Exposure to microplastics increases risk of liver fibrosis
Written by Ryan Murray, PhD Student at the University of Leeds
Ryan Murray, PhD Student at the University of Leeds, summarizes an interesting paper on the effects of environmental microplastics on liver health.
Paper: “Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway.” By Wang et al., 2022, Environmental Pollution. PMID: 35167931.
The deposition of plastics in our ecosystem is climbing at an exponential rate, due to factors such as an increased industrial footprint and the incomplete disposal of plastic waste. These microplastics (<5mm particle size) are now entering our food chain through the ingestion of organisms that contain them. Issues arise over time due to the inability of humans to hydrolyze certain plastics such as polystyrene, which can then undergo bioaccumulation and lead to liver failure. The liver is the epicenter of toxin decomposition and waste disposal in the human body. We are now aware, as shown through transcriptomics, that microplastics induce hepatocyte apoptosis via reactive oxygen species driven by Ca2+ overload in the system. In the human model, the decomposition of microplastics by physiochemical forces is limited due to the chronic damage they induce on the organ in question. For example, in the liver, this induces a scarification process commonly referred to as fibrosis.
Liver fibrosis is characterized by the excessive deposition of extracellular matrix components (ECM) in the areas surrounding hepatic tissues, a process that is tightly regulated by the cyclic GMP-AMP synthase (cGAS) stimulator of interferon genes (STING) inflammatory pathway. This pathway is well accepted to be a potential downstream therapeutic target for inflammatory disease. Mechanistically, the cGAS/STING pathway is directly involved in host immunity to detect and identify foreign DNA by allosterically binding to dsDNA and catalyzing the upregulation of proteins that subsequently leads to the elimination of foreign genes. Wang et al. evaluated the level of disruption caused by the exposure of mouse liver tissue to microplastics at the mitochondrial and nuclear levels. Due to the nature of activation of the cGAS/STING inflammatory pathway, the authors hypothesized that free dsDNA in the cytoplasm of liver cells is destroyed by the presence of microplastics, thus leading to eventual liver fibrosis over time.
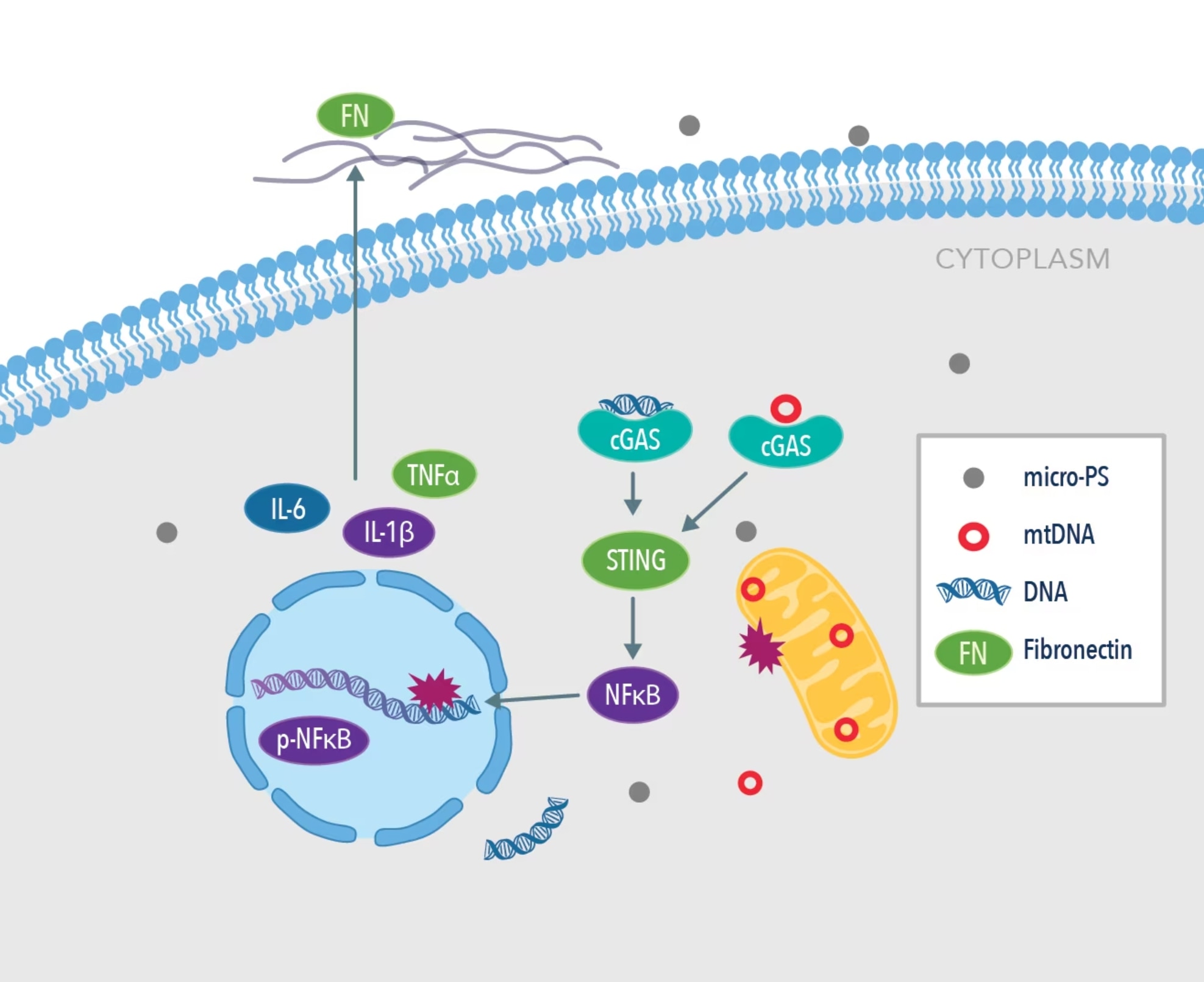
Graphical abstract from Wang et al., 2022 Environmental Pollution. PMID: 35167931. Image adapted from the paper.
The team found a high proportion of microplastics around 0.1 µm in size in the cytoplasm of liver cells in mice, providing evidence that microplastics <1 µm can readily invade the intracellular space. Hepatocellular histopathology revealed that damage to liver cells increased with an increase in the size of microplastics from 0.1 µm to 1 µm. In the microplastics groups, common serum markers of liver damage were raised after 60 days’ treatment, indicating that high levels of oxidative stress may play a pivotal role in hepatocyte damage.
To determine the effect of microplastics on mitochondrial function in the liver, the team observed a downregulation in the expression of PPARα and PGC-1α, two key regulators of mitochondrial biosynthesis. The fission and fusion of mitochondrion directs cell growth, and it was observed that the presence of microplastics downregulated expression of the fusion protein MFN-1 in a dose-dependent manner in HL7702 cells. As expected from their hypothesis, they found that the cGAS/STING pathway was significantly upregulated in the microplastics groups.
It has also been reported in the literature that the inflammatory response drives the fibrosis of the liver tissue to coordinate it with the levels of cellular repair taking place. Immunofluorescent staining revealed that levels of expression of fibronectin were increased in the treated group of mice after exposure to microplastics (1mg/L) in the liver. In healthy liver tissue, expression of fibronectin is low, and an increased expression of this protein is closely related to adverse architectural changes in tissue morphology. The authors also discovered that the common inflammatory markers IL-1β, IL-6, and TNFα expression were higher in the treated groups vs. control. Treatment with C176, a pharmacological inhibitor of STING, promoted the recovery of hepatocytes and alleviated the level of fibrosis induced by microplastics. Histopathological staining revealed the degree of liver pathology in relation to the size and length of exposure to microplastics over a 60-day period. Compared to the control group, mice exposed to 1mg/L PS over 60 days showed a 4-fold increase in their liver pathology score, thus indicating that microplastics may have a detrimental effect on the overall health of the liver tissue.
To conclude, Wang et al. identified that microplastics less than 1 µm in size can enter the bloodstream and are deposited in hepatic cells to then induce a catastrophic downstream inflammatory response. They showed in their models that microplastics promoted nuclear DNA and mitochondrial DNA damage through invasive action, which led to the upregulation of the pro-inflammatory cGAS/STING and increased the deposition of ECM in liver fibrosis.
Proteintech Products
Product Name |
Catalog Number |
| GAPDH | 10494-1-AP |
| cGAS | 26416-1-AP |
| STING | 19851-1-AP |
| Fibronectin | 66042-1-Ig |
| PPARα | 15540-1-AP |
| PGC-1α | 66369-1-Ig |
| MFN-1 | 13798-1-AP |
| DRP-1 | 12957-1-AP |
| ND1 | 19703-1-AP |
| SDHB | 10620-1-AP |
| UQCRC2 | 14742-1-AP |
| COX4 | 11242-1-AP |
| ATP5A | 14676-1-AP |


