B細胞とミトコンドリア:「エネルギー発電所」以上に重要な存在
オックスフォード大学の研究チームは、ミトコンドリアがB細胞の発生およびリンパ腫形成において必須であることを明らかにした論文をNature Immunology誌に発表しました。
紹介論文:Yazicioglu, Yavuz F., et al.「Dynamic mitochondrial transcription and translation in B cells control germinal center entry and lymphomagenesis. Nature Immunology(2023): 1-16.」
Amanda Balboa Ramilo著(ウプサラ大学、PhD candidate)
Bリンパ球(B細胞)は、適応免疫応答に必須の構成要素であり、認識した病原体等の抗原に対し高い親和性を持つ抗体を産生します。B細胞は造血幹細胞を前駆体とする細胞から発生し、成人では骨髄で様々な中間体(プロB細胞、プレB細胞、未成熟B細胞)に分化・成熟します。B細胞はその後胚中心(GC:germinal center)へ移行し、胚中心の暗領域(dark zone)でB細胞受容体(BCR:B cell receptor、膜型免疫グロブリン分子)の可変領域遺伝子に体細胞超変異(SHM:somatic hypermutation)が起こります。次に、B細胞は明領域(light zone)へと移動します。明領域ではB細胞が濾胞性ヘルパーT細胞(Tfh細胞)と相互作用することで、選択的に形質細胞(plasma cell、抗体産生細胞)またはメモリーB細胞(Memory B cell)と呼ばれる各クローンへとさらに分化します。この反応は胚中心反応(germinal center reaction)と呼ばれます。B細胞の分化過程では免疫グロブリンの遺伝子座に急速に変異が生じることから、胚中心反応のプロセスは様々ながんの発生と関連付けられています。例えば、胚中心のB細胞は、非ホジキンリンパ腫の中で最も多い病型である、びまん性大型B細胞リンパ腫の原因となる細胞です。
胚中心のB細胞は急速に増殖し、エネルギー産生の多くを酸化的リン酸化に依存していることが報告されています。この代謝プロセスはびまん性大細胞型B細胞リンパ腫にも存在します。in vitroでは、ミトコンドリアがB細胞のシグナル伝達を制御できることが示されています。しかし、この機構がin vivoでも機能しているか否かは明らかになっていません。T細胞、線維芽細胞、骨髄系細胞のようなその他の種類の細胞では、「TFAM(Transcription Factor A, Mitochondrial、ミトコンドリア転写因子A)」によるミトコンドリアの転写および翻訳が、細胞の機能にとって極めて重要となります。2023年に発表されたオックスフォード大学Kennedy Institute of RheumatologyのYaziciogluらによる論文(2023, Nature Immunology, PMC10232359)は、B細胞の成熟、B細胞が胚中心反応へ移行する際のTFAMおよびミトコンドリアの転写・翻訳の役割を解明することを目的としています。以下に、この興味深い論文の概要を紹介します。
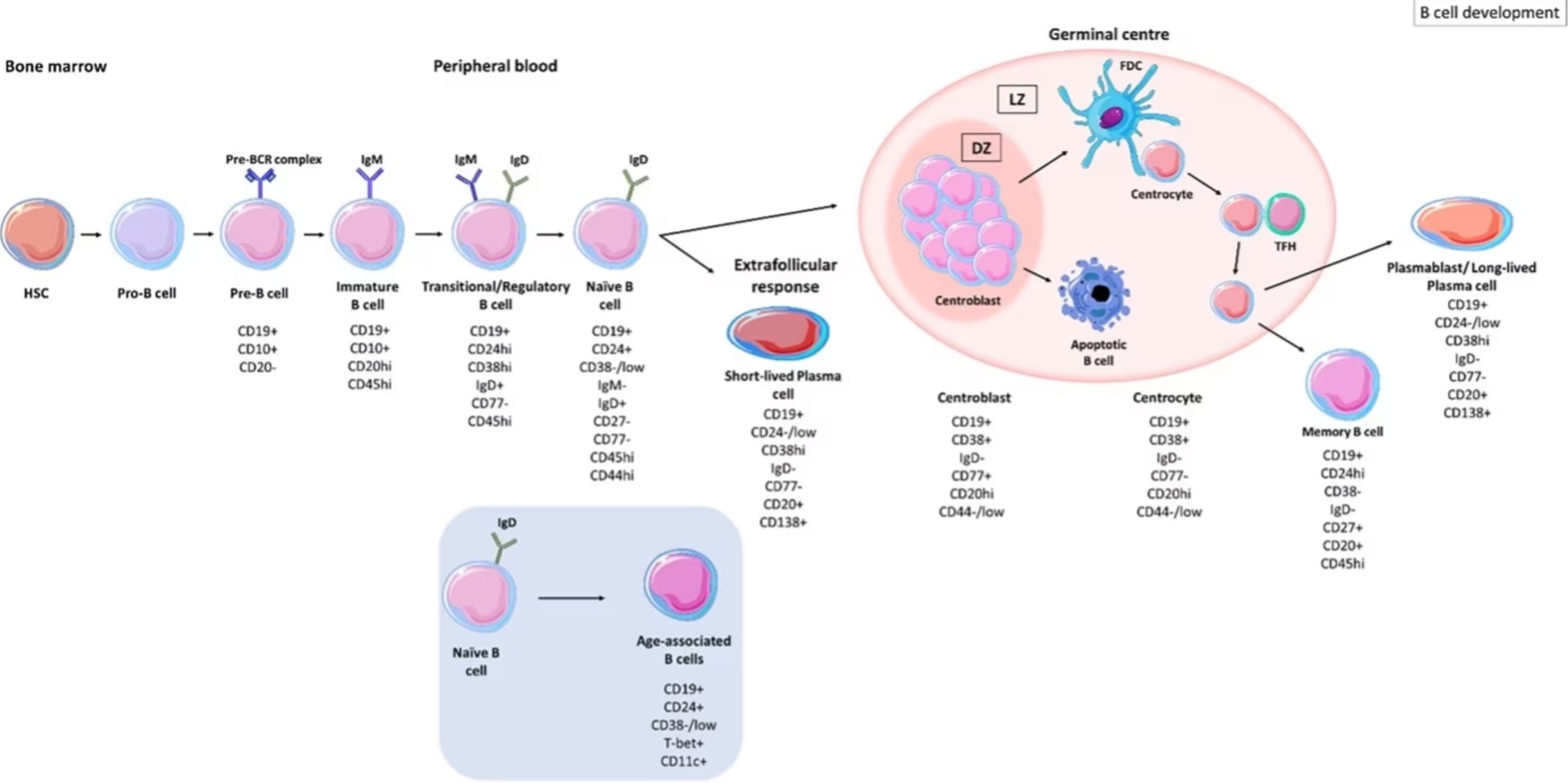
図1. B細胞の発生段階。図は、Patel et al,. 2021, Frontiers of Immunology(オープンソース、PMID: 34630404)より引用。HSC:造血幹細胞、SMH:体細胞超変異、DZ:暗領域、LZ:明領域、FDC:濾胞樹状細胞、TFH:濾胞ヘルパーT細胞。
胚中心B細胞内のミトコンドリアは極めて動的な細胞小器官であり、大きな構造変化を受けます。
胚中心に存在するB細胞のミトコンドリアの構造および密度を焦点顕微鏡およびフローサイトメトリーによって解析した結果、B細胞濾胞周辺に存在するナイーブB細胞と比較すると、胚中心反応に移行したB細胞には融合して大きくなった多数のミトコンドリアが存在することが明らかになりました。胚中心のB細胞はミトコンドリアの質量だけでなく、ミトコンドリアの翻訳・転写レベルも高くなっていました。TFAMはミトコンドリア核様体に発現が認められ、ミトコンドリア翻訳・転写が活発な領域に共局在していました。
一連の結果は、B細胞が胚中心反応へ移行するには、ミトコンドリア量を増加させる必要があり、おそらくTFAMによって刺激されてB細胞のミトコンドリア翻訳・転写速度が加速し、胚中心反応プロセスへの移行が生じることを示唆しています。
TFAMはB細胞内で動的に制御されており、細胞の運動性を調節することでB細胞の分化と胚中心反応への移行に必要であることが示されました。
正常なB細胞の分化におけるTFAMの関連性を評価するために、B細胞の条件特異的TFAM欠損(B-TFAM)マウスを作製しました。フローサイトメトリー解析により、骨髄ではTFAM欠失B細胞は1)プロB細胞の段階からプレB細胞の段階へ移行できず、2)脾臓では、辺縁帯B細胞および濾胞B細胞の合計数が著しく減少することが示されました。高次元フローサイトメトリーによるタンパク質発現解析では、TFAMの欠失によりミトコンドリアにコードされるCOXIの下方制御と、核にコードされるCOXIV、ATP5A1、HSP60の代償的な上方制御が示されました。
これらのタンパク質の検出には、プロテインテックのCoraLite®(コーラライト)標識抗体が用いられています。
COXIV:CoraLite® Plus 488-conjugated COXIV Monoclonal antibody(カタログ番号:CL488-60251)、ATP5A1:CoraLite® 555-conjugated ATP5A1 Monoclonal antibod(カタログ番号:CL555-66037)、HSP60:CoraLite® 594-conjugated HSP60 Monoclonal antibody(カタログ番号:CL594-66041)
胚中心のB細胞から特異的にTFAMを欠失させると(Aicda-TFAM)、免疫感作後に形成される胚中心は数の減少とサイズの縮小が認められ、顕著に無秩序な構造を呈し不十分な細胞コンパートメントを示しました。
免疫感作したAicda-TFAM細胞のシングルセル遺伝子発現プロファイルでは、TFAMを発現しない細胞にはミトコンドリア遺伝子発現の調節不全が生じ、細胞の運動性に関連する遺伝子が最も影響を受けていることが示されました。Aicda-TFAM細胞の転写プロファイルからは、細胞輸送や細胞骨格の動態に変化が生じることが示唆されます。
一連の結果から、著者らはTFAMが正常なB細胞の分化および胚中心への移行に必須の転写因子であることを述べています。TFAMが存在しない場合、B細胞は最終的な成熟状態に到達できず、ミトコンドリアのタンパク質発現に障害が生じます。さらに、免疫感作後のB細胞の組織化や機能的な胚中心を形成する能力が低下します。したがって、TFAMはB細胞の細胞骨格を維持し、B細胞が胚中心へと移動し適切な空間配置に留まるために必須の転写因子であることが示唆されます。
TFAMはリンパ球の分化に必須の転写因子です。ヒトリンパ腫におけるミトコンドリアの転写・翻訳の薬理的阻害は、疾患の有力な治療ターゲットになり得ます。
体細胞超突然変異(SMH)プロセスの過程で発生するびまん性大細胞型B細胞リンパ腫は、一般的にMYC遺伝子が免疫グロブリン遺伝子の遺伝子座と転座することによって引き起こされます。TFAMはB細胞の体細胞超変異が生じる場となる胚中心反応へのB細胞の移行を制御していることから、著者らはTFAMを欠失させることでリンパ腫形成を防ぐことができるのではないかという仮説を立てました。著者らは、リンパ腫のマウスモデルにおいて、B細胞からTFAMを欠失させることでマウスにおけるリンパ腫の発症を効果的に阻止することを実証しました。さらに、リンパ腫細胞はTFAMを発現し、ミトコンドリア翻訳・転写のレベルが高いことを示しました。したがって、ミトコンドリア転写および翻訳の薬理学的阻害が、ヒトリンパ腫の治療ターゲットとなる可能性が浮上しています。この仮説を検証するために、著者らはヒトリンパ腫細胞株のミトコンドリア転写・翻訳を阻害することで細胞増殖が抑制されることを示しました。
結論として、Yaziciogluらによる研究は、TFAMはB細胞においてミトコンドリア活性の増進や細胞骨格構成の維持に働くことによって、B細胞分化に必要とされる制御因子であることを立証しました。TFAMの働きにより、B細胞が胚中心へ移行し、分化、成熟する一連の胚中心反応が可能になると考えられます。本論文でTFAMがB細胞分化における重要な因子として同定されたことにより、リンパ腫形成におけるTFAM研究の扉が開かれ、TFAMは今後の新規治療ターゲット候補としての探索が期待されます。
論文で使用されたプロテインテック製品
- CoraLite® Plus 488-conjugated COXIV Monoclonal antibody(カタログ番号:CL488-60251)
- CoraLite® 555-conjugated ATP5A1 Monoclonal antibody(カタログ番号:CL555-66037)
- CoraLite® 594-conjugated HSP60 Monoclonal antibody(カタログ番号:CL594-66041)
プロテインテックの関連製品:フローサイトメトリー用標識抗体