初心者のためのフローサイトメトリーゲーティング入門
論文掲載も対応可能な、わかりやすいデータを取得できるフローサイトメトリーゲーティングの手順をポイントごとに解説します。
Michelle Belmont著(プロテインテックグループ研究員[修士号])
フローサイトメーターでサンプルを測定したら、次は何をすれば良いでしょうか?ほとんどのフローサイトメーターは、測定中もいくつかの解析を実行できますが、測定後に得られたFCSファイル(フローサイトメトリーの測定データを格納した標準形式ファイル)を解析用ソフトウェアで処理すれば詳細なデータ解析が可能になり、ドットプロットやヒストグラム等を目的に適した形式で保存できます。
フローサイトメトリーにおけるゲーティングとは?
ゲーティング(Gating)とは、詳細な解析をしたい場合や、わかりやすいデータを作成するために、収集したすべてのイベントからあるサブセットを選択するプロセスを指します。通常、ゲーティングでは細胞サイズ・顆粒度(Granularity:細胞内部構造の複雑さ)・各種細胞マーカーの発現といったターゲット細胞の特徴的なデータを利用して、研究対象のターゲット細胞集団を選別します。その他に、死細胞や死にかけている細胞、あるいは複数の細胞で構成され解析には使用できないイベントを除外するといった、データのクリーンアップを行います。完成度の高いパネルの構築に加え、適切なゲーティングを行うことで、実験データをより解釈しやすく論文掲載も可能な形に整えることができます。
ゲーティングの基本的な考え方は、初代細胞でも細胞株でも概ね共通しています。はじめに細胞を大まかにゲーティング(囲い込み)し、母集団から目的の細胞集団を絞り込んでいきます。ゲート内の細胞群は続く解析に使用されますが、ゲートの外側の細胞は除外されます。パネル構成によっては、ゲーティングで目的細胞を絞り込み、その集団内の表現型等が実験条件によってどう変わるかを比較できます。フローサイトメトリーは多くの場合、免疫細胞のマーカーや機能を調べるために実施されます。そこで、本稿では免疫細胞のフローサイトメトリーのゲーティングについて解説します。
ゲーティング(FSC/SSC)
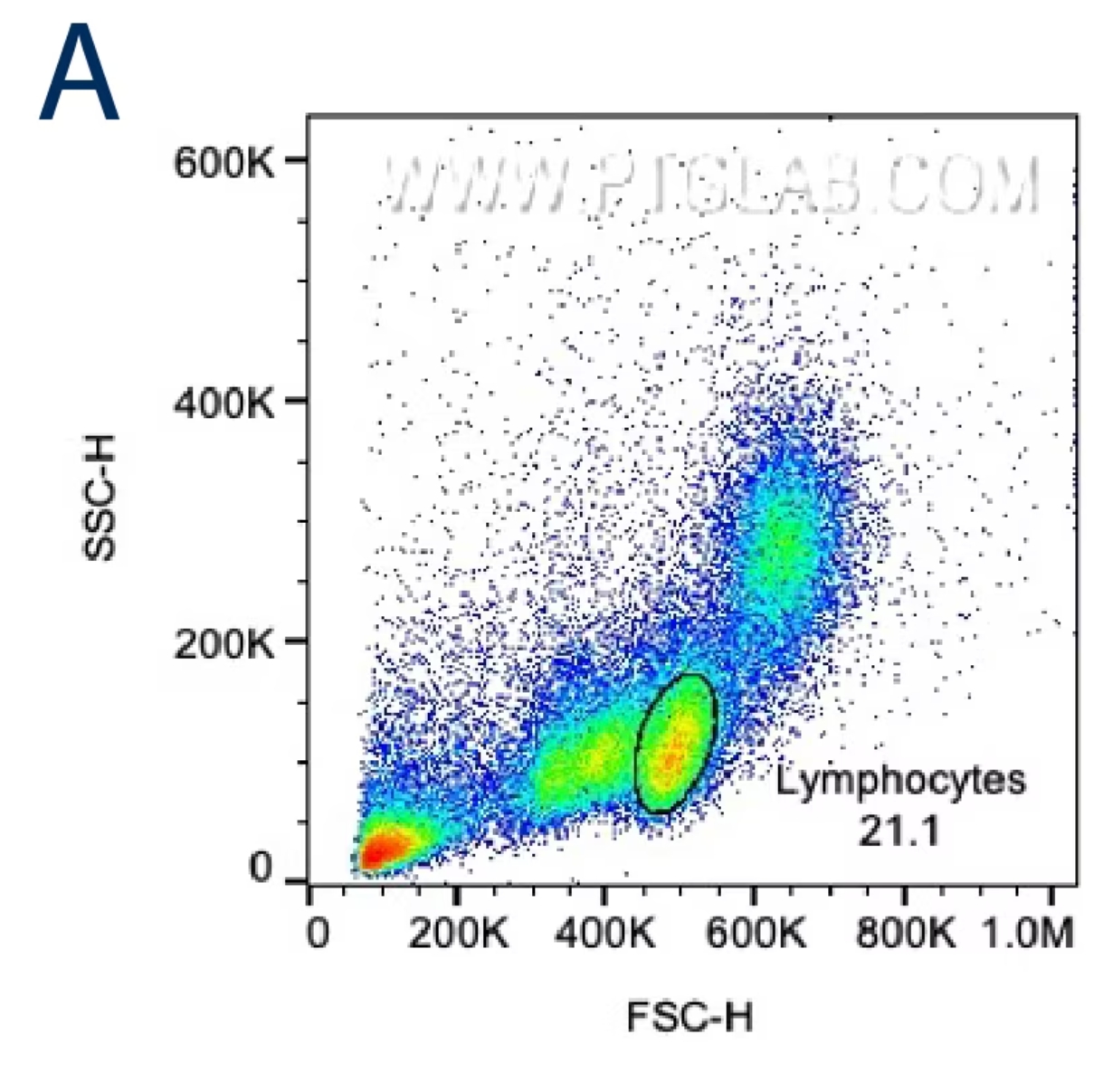
目的の細胞集団を正確に選択するには、まず前方散乱光(FSC:Forward scatter)と側方散乱光(SSC:Side scatter)のプロットを確認します。サイズが大きい細胞はFSC値が高くなり、内部構造が複雑な細胞はSSC値が高くなります。正確なゲーティングを行うために、目的の細胞のサイズと内部構造の特徴を把握しましょう。細胞株を使用して解析する場合、FSC/SSCにより1つの主要な細胞集団が示されるはずです。まずはその集団を囲むようにゲートを設定し、ここから次のゲーティング条件を設定します。末梢血単核細胞(PBMC:Peripheral blood mononuclear cells)や全血サンプル等の初代細胞を解析する場合は、FSC/SSCプロット上のリンパ球・単球、全血の場合はさらに顆粒球等の目的の細胞集団が含まれる領域を大まかに囲い最初のゲートを設定します。
 |
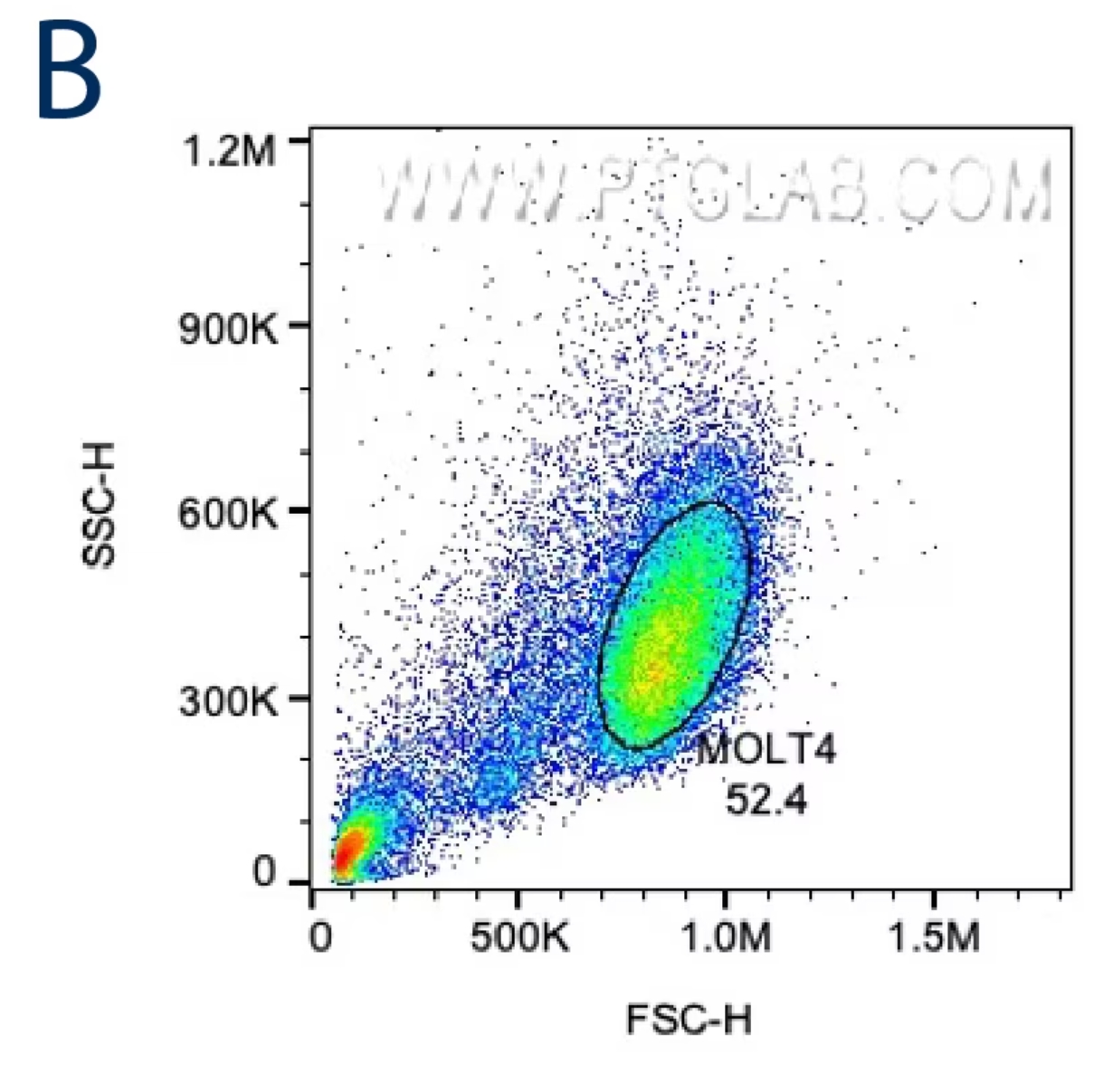 |
図1. A:サイズと粒度に基づきリンパ球をゲーティングするPBMCのFSC/SSCプロット。取得したイベントの21.1%がリンパ球ゲートに含まれています。
B:癌細胞株(MOLT4細胞)のFSC/SSCプロット。取得したイベントの52.4%がこのイニシャルゲートに含まれています。
単一細胞のゲーティング(FSC-A/FSC-HまたはSSC-A/SSC-H)
最初のゲートで選択した細胞をさらに絞り込むには、次にダブレットやトリプレット等の細胞塊を除外します。これは、ダブレットイベント等が存在すると、最終的なゲーティングで得られる結果が歪曲される原因になるためです。細胞塊イベントの除外はFSC-A/FSC-H(またはFSC-W)か、SSC-A/SSC-Hをプロットして実施しますが、一般的にSSC-A/SSC-Hの方がダブレット除去の感度がより高い傾向にあります(A:Area、H:Height、W:Width)。複数の細胞が1つのイベントに含まれる場合、パルス幅の増大等によりピーク総面積(A:Area)がピーク高さ(H:Height)に対して相対的に大きくなることが多く、単一細胞(シングレット)以外のイベントとみなされます。また、ピーク幅(W:Width)を高さの代わりに使用してもダブレットを除去できます。単一細胞の場合、シグナルの幅と高さは面積に比例し、シングレットのイベントはピークの面積/高さまたは面積/幅をプロットしたグラフの対角線にあたる領域に分布しますが、複数の細胞からなるイベントはこの対角線から外れた位置に分布します。場合によっては、目的の細胞を大まかにゲーティングする前にダブレット除去を実施することもあります。
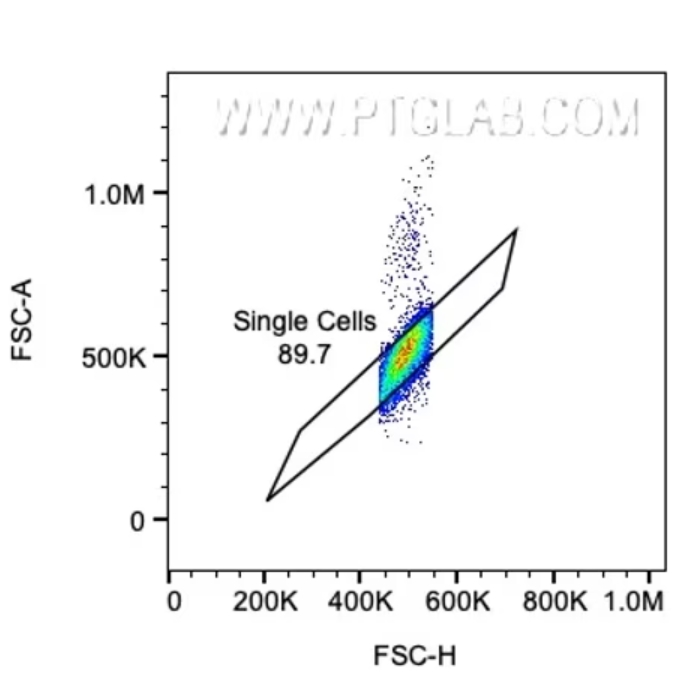 |
図2. FSC-A/FSC-Hプロットによるゲーティングで、グラフの対角線付近に分布する単一細胞(シングレット)を囲い込みます。
生細胞のゲーティング
分析対象を単一細胞のみに設定した後は、生細胞をゲーティングします。死細胞は非特異的な吸着を起こしやすく、本来結合しない抗体や蛍光色素が結合してしまう可能性があるため、不正確な結果が出たり、結果を誤って解釈する原因となります。さらに、死細胞は蛍光強度や散乱特性が不均一になり、シグナルの面積や高さの値がばらつき、解析に含めることでデータの質が低下する場合もあります。パネルデザインによっては、チャンネルに別の蛍光シグナルの漏れ込み(スピルオーバー)が生じる可能性があるため、生細胞のゲートは余裕を持たせた設定にする必要がある場合があります。結果に死細胞が含まれている場合や、生細胞が除外されていた場合は、いつでも生細胞ゲートの適用段階に戻りゲート条件を再調整することができます。細胞の生死を判別するための蛍光色素は、フローサイトパネル内の他のマーカーとは異なるチャンネルに設定するよう留意してください。
細胞の生死判別用の蛍光色素の選び方については、ブログ「To dye or not to dye?」をご覧ください。
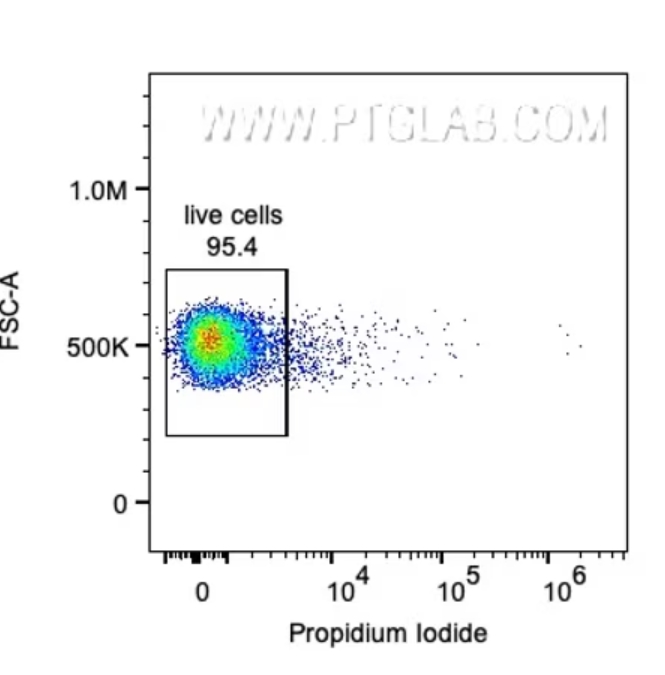 |
図3. 生細胞のゲーティング。ヨウ化プロピジウム(PI:Propidium Iodide)陰性の生細胞をゲーティングしています。
目的の細胞を解析する
上述したゲーティングによって、目的の生存単一細胞だけのデータが得られるはずです。続いては、パネル内のマーカー発現パターンを確認します。パネルデザインは明瞭かつ正確な結果を得るために重要で、目的の細胞集団のマーカー発現や共発現に合わせてデザインを最適化します。パネル内の様々なマーカーと蛍光色素の組み合わせを検討する際に参考になる情報を、FluoroFinder等のツールが提供しています。
目的細胞を解析する際は第一に、より広範に発現するマーカー(例:白血球のCD45)を選択し、その次に、より細胞特異的なマーカーを使用して目的の細胞集団が得られるまで細胞集団を絞り込むと良いでしょう(例:CD8+細胞傷害性T細胞の場合はCD3+/CD19-/CD8+をマーカーとして選択します)。その後、絞り込まれた特定の細胞集団が発現する様々な細胞固有のマーカーを解析します。一例を挙げると、CD8+細胞傷害性T細胞は多くの場合、活性化するとインターフェロンガンマ(IFN-γ:Interferon gamma)を分泌します。解析時のパネルにIFN-γを追加することで、得られたCD8+細胞傷害性T細胞集団におけるIFN-γの産生を確認することができます。
ゲーティング時の細胞集団の分布を把握しやすくするために、プロットの表示を調整できる機能がいくつも用意されています。ゲートをより正確に実施するためにX軸やY軸の設定を変更すると、陽性細胞集団と陰性細胞集団を視覚的にわかりやすく区別することができます。さらに、サイズの大きな細胞や蛍光シグナルが強い細胞は、設定された軸の範囲の最大値を超えてしまう場合がありますが、軸の最大値と最小値を調整することで表示範囲外にあったイベントもプロット上に適切に表示できます。また、「スムージング(smoothing)」ツールを使用して、ドットプロットの分布を見やすく整えることもできます。その他に、ドットプロットも等高線プロットも、イベントの密度に従って各イベントを色分け表示できます。このような編集機能を使用すると細胞集団が明確になり、より正確に目的の細胞をゲーティングすることができます。ゲーティング・軸調整・スムージング等のツールはヒストグラム形式のデータにも適用できますが、ヒストグラム上で設定したゲートをドットプロットに表示することはできません。
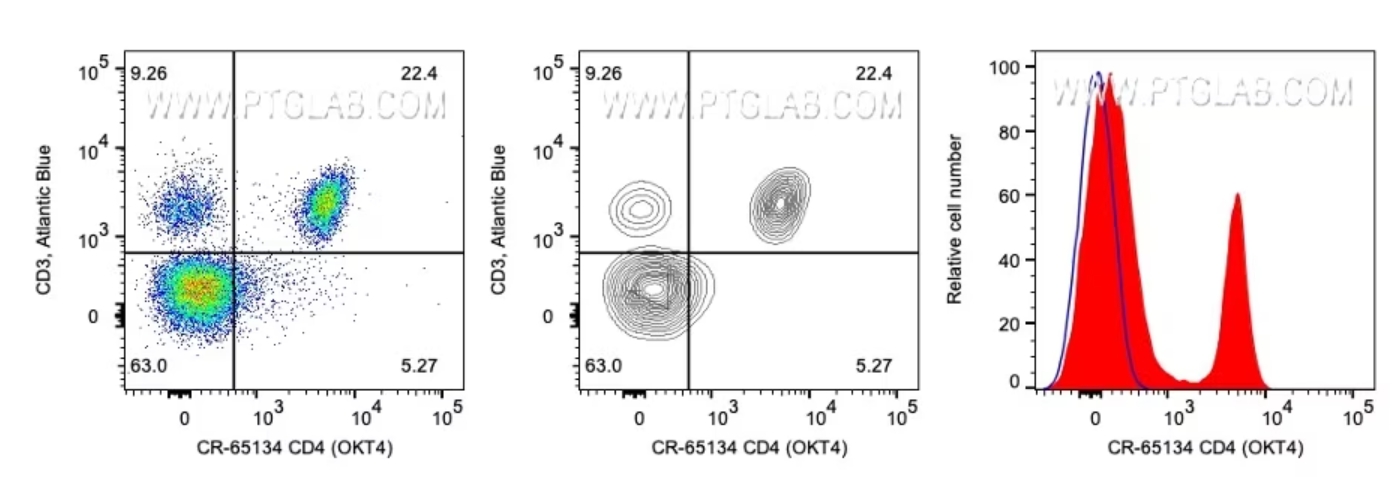 |
図4. 左:CD3(Y軸)およびCD4(X軸)で染色したリンパ球。イベント密度を色分けしたドットプロット形式で表示。使用抗体:CD3抗体(カタログ番号:AB-65133)およびCD4抗体(カタログ番号:CR-65134)。 中央:左側と同じデータを等高線プロットで表示したグラフ。 右:同一リンパ球細胞集団におけるCD4発現分布を示すヒストグラム。
ある1つのサンプルで解析対象を絞り込めるゲーティング設定を確立できたら、ワークスペース内のすべてのサンプルにその設定を適用し、すべて一貫した基準でゲーティングを行うことでデータの正確性を確保します。一般的には、解析時のバイアスを防ぐため、FMO(Fluorescence minus one)コントロールを用いてゲートを設定することが推奨されます。FMOは多色解析において蛍光補正後のデータの拡がりを確認するために、評価したい蛍光だけを1色抜いたコントロールです。FMOで陽性に見えるイベントや陰性集団の分布を確認し、蛍光の漏れ込み等に起因するデータの拡がりを考慮したゲートを設定することで、より正確な解析が可能になります。1つのゲーティング設定を複数のサンプルに適用する場合、目的の細胞が意図せず除外されないよう留意し、常に確認する必要があります。FCSデータのゲート設定はいつでも変更可能で、細胞集団をより明確に分離することができます。一連のゲーティングによって、フローサイトメトリーのデータ解析の第一段階が完了します。

フローサイトメトリーをこれから始める方もご安心ください。プロテインテックのフローサイトメトリーパネルは、初心者にも扱いやすく目的の細胞を検出しやすいよう最適化されたパネルです。各パネルは5種類の通常サイズ抗体で構成され、包括的なゲーティング戦略のもとに各種細胞集団を分離・検出することができます。
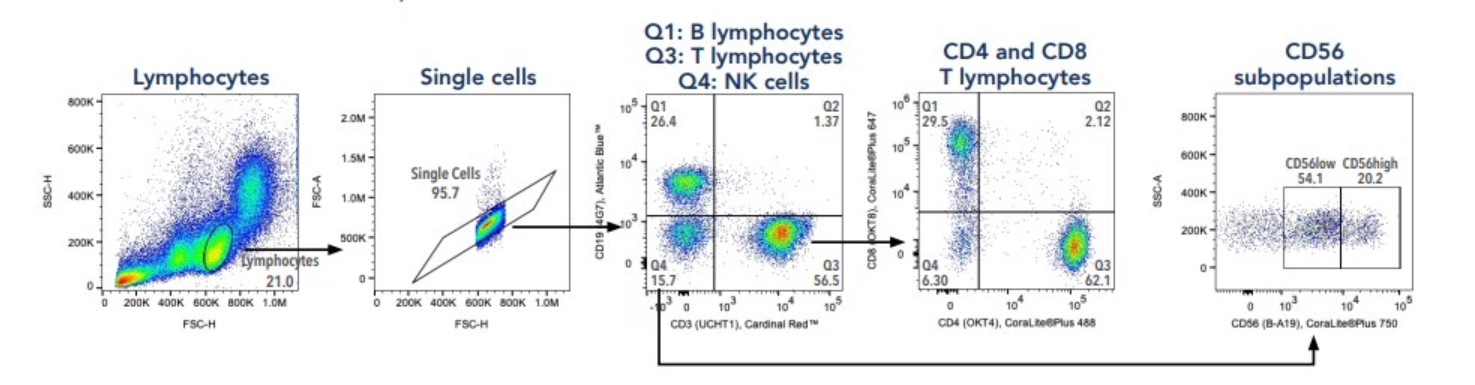
図5. Human TBNK Basics Panel(カタログ番号:PK30012)を使用した、ヒトPBMC由来のT細胞・B細胞・NK細胞を分離するゲーティング戦略と各ターゲット細胞集団。


