構造生物学研究におけるNanobody(ナノボディ)
George Biggin著(マンチェスター大学、専攻分野:構造生物学、博士課程在籍)
ラクダ科動物由来の重鎖抗体から作製されるNanobody(ナノボディ)は、構造生物学研究の現場で基盤ツールとしての存在感を急速に高めています。
本稿では、Nanobodyがどのように利用されているのか、以下の項目について解説します。
- 構造変化しやすいタンパク質の安定化
- 一過性のタンパク質複合体の安定化
- タンパク質精製の効率化
- 見かけの粒子サイズを増大させる(cryo-EM*)
- 擬対称構造の形成回避による解析精度改善(cryo-EM)
*cryo-EM(Cryo-electron microscopy):クライオ電子顕微鏡、低温電子顕微鏡
Nanobodyとは?
Nanobody(Nb)は、分子量が約12~15 kDaのラクダ科動物の重鎖抗体の可変領域の組換え体です(図1a)。この重鎖抗体は、ラクダ、ラマ、アルパカ等のラクダ科動物が産生します。現在、Nanobodyはいくつかの有益な特性を備えることから、従来型抗体に代わる望ましい代替ツールとなりつつあります(図1b)。ラクダ科動物由来のNanobodyは、アフィニティ捕捉試薬や蛍光イメージング用試薬といった学術研究用試薬としてだけではなく、診断または治療目的でも使用されています。Nanobodyは様々な分野で活用されていますが、利用可能なアプリケーションの中でも過小評価されがちなのが、構造生物学におけるその有用性です。
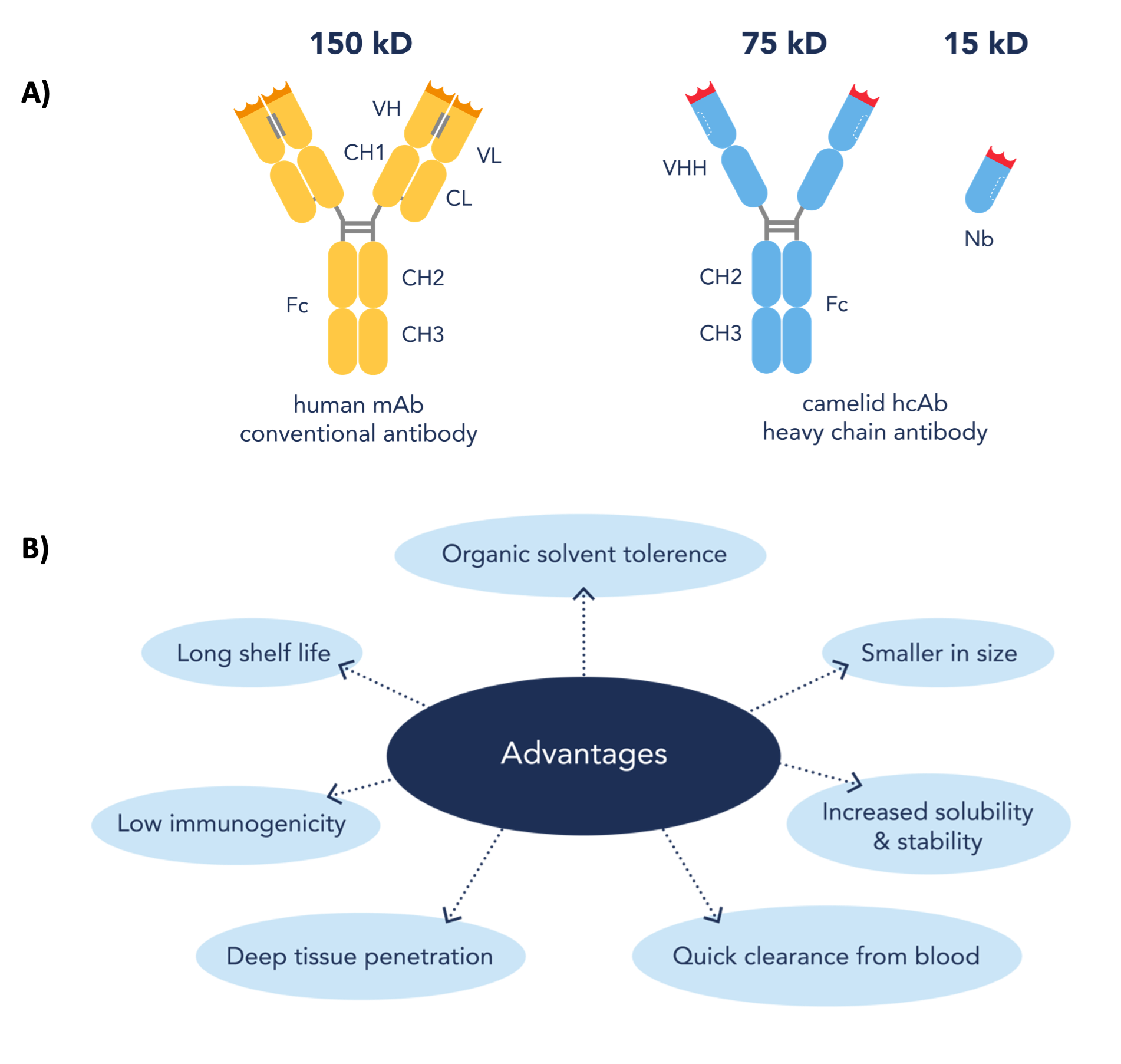
図1. (A)従来型抗体、ラクダ科動物重鎖抗体、ラクダ科動物重鎖抗体のVHH領域の組換え体(Nanobody)のドメイン構造(B)従来型抗体と比較したNanobodyの利点(Nanobodyドメインの図は参考文献[1]をもとに作図)
X線結晶構造解析におけるNanobody
構造生物学は、分子レベルでタンパク質を評価することで、タンパク質構造と機能の関係を原子レベルの分解能で解明することができる研究分野です。X線結晶構造解析は、原子レベルの分解能でタンパク質構造を得るために使用される最も一般的な手法の1つです。しかし、ある種のタンパク質の構造を高い分解能で解明するのは極めて困難な場合があります。様々なコンホメーションを示すタンパク質や、本質的に様々な構造を取りやすい領域を有する/非構造領域を有するタンパク質といった構造変化しやすいタンパク質は、同じ構造のタンパク質が規則的に配置される結晶構造を形成しにくいため、タンパク質結晶の作製には困難を要します。そのため、多くの研究者が、結晶化に関する問題を解決するためのツールの開発に注力しています。そのような一連のツールの中でも、成功を収めているものの1つがNanobodyです。
結晶化シャペロンとして使用するべく、ターゲットタンパク質を認識するNanobodyを作製する方法が多くの研究で示されています[2]。これまでに、Nanobodyシャペロンを使用することで、膜貫通型アンジオテンシンⅡ1型受容体(AT1R)のような結晶化しにくい構造のタンパク質の結晶構造を高い分解能で得ることに成功しています。Cell誌に掲載されたWinglerらによる論文では、酵母ディスプレイ法による合成ライブラリーを使用してAT1Rを認識するNanobodyを作製しています。著者らは、受容体の細胞内トランスデューサーポケットと結合することで、活性化に伴い大きく構造変化するGタンパク質共役型受容体(GPCR)に特徴的な領域を安定化させて、AT1Rを結晶化しやすくする働きのあるNanobodyの作製に成功しました[3]。
プロテインテックの関連製品:アンジオテンシンⅡ1型受容体抗体
その他にも、Nanobodyは一過性のタンパク質複合体を安定化させる働きによって、様々な膜貫通型タンパク質構造の高分解能観察に多大な成果を上げています。Nature誌に報告された画期的な論文において、RasmussenらはGsヘテロ三量体と複合体を形成したβ2アドレナリン受容体(β2AR)と結合するNanobodyの作製に成功しています[4]。詳細な特性解析の結果、β2AR-Gs複合体と結合することで、GTPγSの働きによるβ2AR-Gs複合体の解離を防ぐNanobodyを特定しました。そして、このNanobodyとβ2AR-Gsが結合した複合体を使用してタンパク質結晶を作製し、活性化状態におけるβ2ARの構造を3.2 Åの分解能で報告しました。
プロテインテックの関連製品:β2アドレナリン受容体関連製品
現在、NanobodyはArp4-Nアクチンヘテロ二量体の事例[5]が示すように、生理学的に重要な状態にあるタンパク質の構造決定を推進する有用なツールとして、ますます注目されています。Knollらは、Arp4-Nアクチンヘテロ二量体と結合するNanobodyを作製し、Arp4-Nアクチンヘテロ二量体を含むサブユニットからなるクロマチン関連複合体に結合するNanobodyの同定に成功しました。続いて実施した結晶構造解析により、Nanobodyが結合したArp4-Nアクチンヘテロ二量体の構造を高い分解能で取得することに成功し、正確な結合エピトープが明らかになりました。その後の詳細な構造解析によって、Nanobodyは、Arp8がとる構造と類似した、Arp4とアクチンが形成する構造を認識することが確認されました。
膜貫通タンパク質は、不安定で柔軟性に富み、疎水的な特性を有するため、その精製と構造解析には特に困難を伴います。このような膜貫通型タンパク質の結晶を作製する場合は、水溶液中でも安定性が保たれるように、通常とは異なる界面活性剤やバッファー組成で実験を行う必要があります。膜貫通型タンパク質の精製条件が最適化されることで、結晶作製後の構造解析を成功させる確率を高めることができると考えられます。プロテインテックのVHH(NANOBODY®)試薬は、Gタンパク質共役型受容体クラスAに属するカンナビノイド受容体CB1のような膜貫通型タンパク質の精製を支援する効果的なツールであることが認められています(ホワイトペーパー[PDF(言語:英語)])。構造研究で、単分散状態にあるタグフリーの精製膜貫通型受容体タンパク質を得るには、プロテインテックのNano-Trap(ナノトラップ)シリーズの製品が役立ちます。Nano-Trapには、GFPを含むタンパク質精製用タグを認識するVHH抗体(NANOBODY®)が利用されています。
クライオ電子顕微鏡におけるNanobody
この20年間で、電子顕微鏡の検出技術と画像処理プロセスは共に進歩を遂げました。そのため、クライオ電子顕微鏡(cryo-EM:Cryo-electron Microscopy)を使用して明らかにされた高分解能タンパク質構造の報告数が急速に増加しています。本手法は厳密な分子工学的手法を使用せず、必要なサンプル量も少量で済むため、現在多くの構造生物学研究者に好まれている手法です。さらに、溶液中のタンパク質サンプルを急速に凍結させるため、X線結晶構造解析と比較すると、よりネイティブに近い状態でタンパク質構造を決定できます。
しかし、一過性のタンパク質複合体の相互作用を調べる場合や、コンホメーション変化するタンパク質、構造変化しやすい領域を有するタンパク質を構造解析する場合、サンプルの安定性や不均一性を原因とする問題が発生することがあるため、クライオ電子顕微鏡での解析は特に困難を伴います。Nanobodyは分子量が小さいため、かつてはcryo-EMのアプリケーションとして見落とされていましたが、現在はcryo-EM観察に伴ういくつかの問題を克服するツールとして研究が進められています。
特に、cryo-EMに供するタンパク質の柔軟性やタンパク質複合体の安定化に関する問題の克服にNanobodyは成果を上げています。Structure誌(Cell Press)に掲載された論文では、研究者らがNanobodyを利用して、菌類(Chaetomium thermophilum)のctTel1の高分解能構造の取得に成功しています[6]。このctTel1は、キナーゼであるヒトATM(Ataxia telangiectasia mutated)/Tel1の相同分子種に相当するタンパク質です。ATMは活性化すると、DNA損傷に対する細胞応答に関与します。この研究では、ctTel1のN末端フラグメントを認識するNanobodyが作製されました。特性解析を実施した後、いくつか作製した中から選択した2種類のNanobodyのうち1つを内因性ctTel1の免疫アフィニティプルダウンに利用し、もう1つのNanobodyはctTel1と複合体を形成させてN末端領域を安定化させるために利用し、最終的な高分解能構造の取得に利用しました。
プロテインテックの関連製品:ATM抗体
プロテインテックのプルダウン精製試薬:Nano-Trap(ナノトラップ)
Nanobodyはcryo-EMにも利用されており、単粒子解析(SPA:Single particle analysis)時に、解析に適した配向で氷に包埋したタンパク質を得る場合や、粒子をアライメントさせたい場合に生じる諸問題を克服します。解析用の試料を作製する場合、タンパク質を非晶質の氷の中にランダムな配向で包埋するのが理想的です。タンパク質の高分解能三次元構造を取得するには、ランダムな配向の多数の粒子を解析することが極めて重要となります。しかし、ある種のタンパク質では、表層構造が原因となってタンパク質の特定の領域が気液界面へ優先的に吸着することがあります。GABA受容体はこのような事例に該当するタンパク質で、LavertyらはGABA受容体のα1サブユニットを認識するNanobodyを作製し、このNanobodyをピロリ菌の細胞外接着ドメインに融合させた、GABA受容体タンパク質の粒子をアライメントさせるタンパク質の足場となる「megabody(メガボディ)」を作製しました[7]。研究者らは作製したメガボディを使用して、cryo-EMのデータ収集とデータ処理に関する問題を克服し、3.2 Åの分解能でGABA受容体の構造を報告することに成功しました。
プロテインテックの関連製品:GABRA1抗体
Nanobodyは、このようなメガボディの構成要素となることで、観察したいタンパク質の粒子サイズを増大させ、タンパク質が気液界面に優先的に吸着しないようにするために使用されています。Nanobodyはそれ以外の用途でもcryo-EMに使用されており、ABCトランスポーターのようなタンパク質の擬対称構造をとるヘテロ二量体の片方のタンパク質に結合させることで単粒子解析の精度を改善し、高分解能でタンパク質を再構成させた結果が得られています[8]。
CLEMにおけるNanobody
光-電子相関顕微鏡法(CLEM:Correlative Light and Electron Microscopy)は、蛍光顕微鏡と電子顕微鏡を組み合わせた急速に普及しつつある手法です。CLEMでは、細胞の蛍光イメージングを実施して特定のタンパク質の局在を空間的・時間的に高い解像度で計測し、電子顕微鏡を併用することで、タンパク質の構造を超微細構造レベルで明らかにします。Nanobodyを利用したツールは存在量の少ないタンパク質をCLEMで検出する際に使用された実績があり、近年では特定のタンパク質相互作用の局在を検出するために使用されています。Martellらは、「APEX(Enhanced APX)」と命名した新規アスコルビン酸ペルオキシダーゼ(APX)タグを開発して、APEXを観察対象のタンパク質に融合して細胞内に発現させて、顕微鏡画像を撮影することに成功しました。細胞を固定し、DAB(ジアミノベンジジン)溶液を塗布してH2O2を添加すると、APEXがDABの酸化重合を触媒してDABポリマーの架橋沈殿物が生成されます。このDABポリマーを高電子密度のOs(オスミウム)で染色してコントラストを高め、電子顕微鏡で観察しました[9]。この研究内容をもとにして、Ariottiらは、遺伝子組み換え技術を用いてAPEXに抗GFP Nanobodyを融合しました。研究対象となるGFPタグ融合タンパク質と結合しないAPEX-Nanobodyはプロテアソームによって分解され、目的タンパク質と結合したAPEXのみがシグナルを生成するため、シグナルノイズ比(S/N)が劇的に改善されます。このAPEX-NanobodyをGFPタグ融合タンパク質存在下で発現させることで、CLEMを用いて目的タンパク質の局在を高いシグナルノイズ比で測定することができます。このようなAPEX-Nanobodyツールは、二分子蛍光補完(BiFC:Bimolecular fluorescence complementation)法を実施するために開発が進められており、細胞内タンパク質相互作用の検出と局在の確認を超微細構造レベルで実現します[10]。
プロテインテックの関連製品:未標識VHH抗体(NANOBODY®)
マンチェスター大学で構造生物学研究に用いられるNanobody
現在実施中のマンチェスター大学の研究では、TGFβのバイオアベイラビリティを制御し、活性化する機構におけるフィブリリン1(Fibrillin 1)の役割を解明する取り組みが進められています。フィブリリン1は細胞外マトリックスタンパク質であり、ミクロフィブリル(Microfibril)に取り込まれます。ミクロフィブリルは、弾性線維の形成に極めて重要な役割を果たし、組織に柔軟性と伸展性を与えるため、様々な組織が機能するうえで欠くことのできない構造体です。フィブリリン1遺伝子が変異すると、マルファン症候群に認められるように異常なフィブリリン1が産生・分泌され、TGFβシグナル伝達に異常が生じることによって、全身の結合組織が脆弱になります。
プロテインテックの関連製品:Fibrillin 1抗体
TGFβ抗体
プロテインテックの関連情報:TGF-betaパスウェイポスター[PDF(言語:英語)]
cryo-EMを使用してフィブリリン1とTGFβ複合体の構造を決定すれば、TGFβの活性化制御に隠された分子メカニズムに関する知見が得られると考えられます。しかし、フィブリリン1はビーズが数珠つなぎになったような形状をとり、柔軟性に富む固有の構造を有するため、構造決定には困難を伴います。現在進められている実験では、リンカーで繋いだ2分子のNanobodyとフィブリリン1を共発現する試みに取り組んでいます。フィブリリン1にNanobodyを結合させると、Nanobodyがクランプのような役割を果たしてフィブリリン1の柔軟性を減衰させ、cryo-EMによる構造決定ができるのではないかという仮説が立てられています(図2)。

図2. Nanobodyを利用してフィブリリン1を固定化する模式図。柔軟性に富むフィブリリン1の構造変動を抑制し、構造解析に適した状態に整えます。
最後に
端的に言えば、Nanobodyの登場は構造生物学の分野に革命をもたらし、複雑な生体システムの解明を進展させる新たな可能性を提供しています。膜タンパク質、構造変化しやすいタンパク質といった構造解析が困難なタンパク質をターゲットとする、分子量が小さく安定性の高い汎用性に優れた試薬は、X線結晶構造解析、クライオ電子顕微鏡(cryo-EM)、光-電子相関顕微鏡法(CLEM)等の様々な構造解析技術においてその有用性を発揮しています。Nanobodyの力を活用することで、研究者は構造生物学やバイオテクノロジー、さらにはその他の分野においても、未開拓の領域を解明していくことができるでしょう。
プロテインテックのVHH(NANOBODY®)試薬
プロテインテックは、高品質で信頼される製品を提供することで抗体試薬業界を常に牽引しています。その中でもVHH(NANOBODY®)製品の提供は研究支援の選択肢を一段と拡げました。Nanobodyという優れたツールに対する需要の高まりを鑑みて、プロテインテックは免疫蛍光染色(IF)、免疫沈降(IP)、生物物理学的解析、タンパク質精製等の様々な研究ニーズに応えるChromoTek VHH(NANOBODY®)試薬の包括的な製品拡充に取り組んでいます。
プロテインテックのVHH(NANOBODY®)試薬
免疫蛍光染色‐Nano-Booster & Nano-Label(ナノブースター&ナノラベル)
免疫沈降‐Nano-Trap(ナノトラップ)
生物物理学的解析‐Nano-CaptureLigand™(ナノキャプチャーリガンド)
タンパク質精製‐Spot-Tag® 捕捉/検出システム
高い精度で構築された一連のVHH(NANOBODY®)製品は、ターゲットに対する高い親和性と特異性を備えています。プロテインテックのVHH(NANOBODY®)製品は、シグナル伝達経路の研究、タンパク質間相互作用の探索、より進んだ構造生物学解析技術等の研究ニーズに適した最先端のツールを提案します。
プロテインテックでは、GFP-Trap®のサンプルを無償で提供しています。下記フォームよりご依頼ください。
(※ 国内におけるプロテインテック製品の出荷および販売は、コスモ・バイオ株式会社を通じて行っております。
最寄りのコスモ・バイオ株式会社 代理店をご指定の上、ご依頼ください。)
Nanobodyを利用した試薬をお求めの際は、ぜひプロテインテックをご利用ください。
参考文献
- P Bannas, J Hambach, F Koch-Nolte. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Front Immunol. 2017 Nov 22:8:1603.
- C McMahon, et al. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat Struct Mol Biol. 2018 Mar;25(3):289-296.
- LM Wingler, C McMahon, DP Staus, et al. Distinctive Activation Mechanism for Angiotensin Receptor Revealed by a Synthetic Nanobody. Cell. 2019 Jan 24;176(3):479-490.e12.
- SGF Rasmussen, BT DeVree, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011 Jul 19;477(7366):549-55.
- KR Knoll, S Eustermann, et al. The nuclear actin-containing Arp8 module is a linker DNA sensor driving INO80 chromatin remodeling. Nat Struct Mol Biol. 2018 Sep;25(9):823-832.
- M Jansma, C Linke-Winnebeck, S Eustermann, K Lammens, et al. Near-Complete Structure and Model of Tel1ATM from Chaetomium thermophilum Reveals a Robust Autoinhibited ATP State. Structure. 2020 Jan 7;28(1):83-95.e5.
- D Laverty, et al. Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer. Nature. 2019 Jan;565(7740):516-520.
- S Hofmann, D Januliene, AR Mehdipour, et al. Conformation space of a heterodimeric ABC exporter under turnover conditions. Nature. 2019 Jul;571(7766):580-583.
- JD Martell, et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol. 2012 Nov;30(11):1143-8.
- N Ariotti, J Rae, et al. Ultrastructural localisation of protein interactions using conditionally stable nanobodies. PLoS Biol. 2018 Apr 5;16(4):e2005473.
* NANOBODY®は、Ablynx N.V.社の登録商標です。
免疫沈降用アフィニティビース
<Nano-Trap 無料サンプル配布中!>
関連情報
ペプチドタグ「Spot-Tag®」とVHH抗体(別名:NANOBODY®)による捕捉/検出システム(ブログ)