Quartz Crystal Microbalance: Next-generation biosensor?
Understand how the Quartz Crystal Microbalance technique could be the future of biosensing and biomarker detection.
Written by Benjamin Raven, Biochemistry PhD Student at the University of Sheffield, UK.
Material science and nanotechnology can have monumental applications in biology, where manipulating and understanding matter at the nanoscale helps lead to ground-breaking innovations and discoveries. The Quartz Crystal Microbalance (QCM) technique is a remarkable tool that enables scientists and researchers to delve into the molecular dynamics of surface interactions. The QCM technique operates at the crossroads of physics, chemistry, and engineering, offering a unique window into the realm of molecular binding, adsorption, and thin film deposition. This method has revolutionised our ability to scrutinise and quantify the most subtle changes in mass on a sensor surface, providing insights that extend from fundamental scientific inquiries to cutting-edge applications in biosensing, material coating, and beyond.
What does Quartz Crystal Microbalance do?
QCM is a technique used to measure mass change on a sensor surface. While QCM has been used since the 1960s to measure surface thickness and mass changes, it is only relatively recently that the technique has been exploited for molecular biological analysis. The 1985 paper titled ‘Experimental aspects of use of the quartz crystal microbalance in solution’ by Stanley Bruckenstein and Michael Shay [1] paved the way for the use of QCM for the measurement of biological specimens. Before this point, QCM techniques required a vacuum. However, introducing a liquid phase to the sensor's surface allows a biological analyte to be passed over the sensor, and the binding of the analyte to the functionalised QCM surface measured.
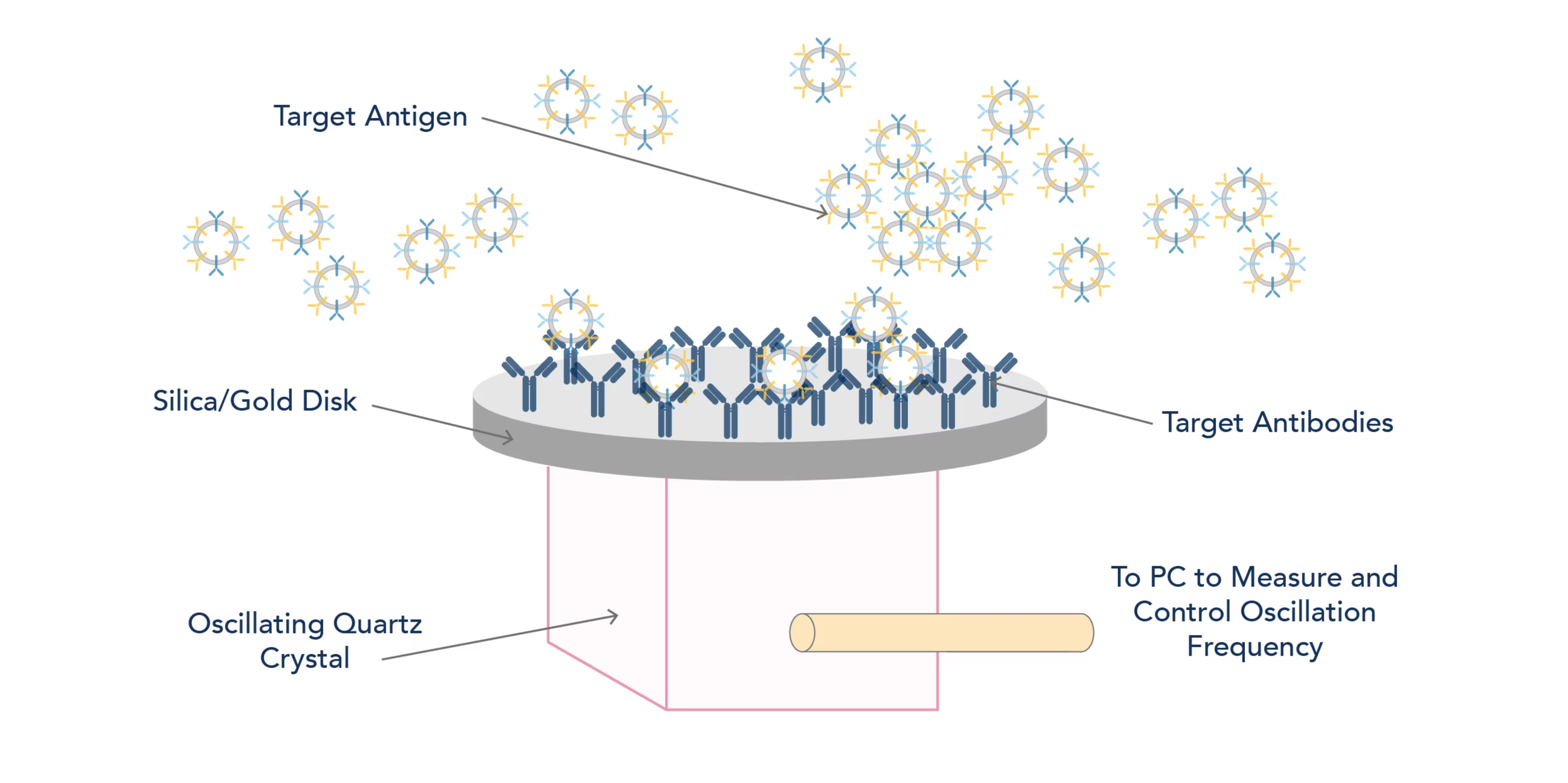
Figure: Diagram detailing the quartz crystal microblance technique.
What is the principle of Quartz Crystal Microbalance?
QCM exploits the piezoelectric effect some materials, such as Quartz crystals, possess. The piezoelectric effect is the phenomenon where certain materials generate an electric charge when exposed to mechanical stress. This can be used to generate electricity from mechanical input, as seen by electric BBQ lighters and mechanical strain gauges used to measure building deformation. However, the piezoelectric effect is reversible, meaning that voltage can induce oscillation in the piezoelectric material. This has applications in timekeeping (the quartz watch uses an oscillating quartz crystal to keep time accurately) and in internal computer clocks. Many pieces of scientific equipment that require a finely controlled oscillation (such as AFM and QCM) also use the piezoelectric effect. QCM works by using the piezoelectric effect to oscillate a functionalised surface at a set frequency (usually 5MHz). Any mass changes will alter the frequency that the system is oscillating at, and these frequency changes can be measured and the resulting mass change calculated.
How does the Quartz Crystal Microbalance work?
The fundamental physics of QCM is that the oscillation frequency of the system is proportional to the system's mass (the Sauerbrey equation). Should a component of the analyte bind to the sensor surface, such as through antigen-antibody binding, this will change the mass of the sensor. While small, this mass change will result in a measurable frequency change in the system. This frequency change can be mapped, and a mass change can be calculated by using the Sauerbrey equation (modified for use in liquid). This calculation can be done quickly using coding languages such as Python, as many of the factors in the Sauerbrey equation are constant (e.g. density and shear modulus of quartz). While QCM does not directly measure mass (instead measuring frequency of oscillation), the technique can be used as a way to calculate mass change on a surface. This mass change can be caused by thin film deposition [2], erosion [3] or by the binding of an analyte to the sensor surface. This can confirm whether your sample has antigens present for the target antibody that the QCM sensor surface is functionalised for.
Quartz Crystal Microbalance for biomarker detection
QCM is an especially powerful technique for biomarker discovery and validation. By functionalising the QCM sensor surface with Streptavidin, biotinylated antibodies can be orientated (N-terminus facing up) and bound to the surface. This orientation improves sensitivity by ensuring that the N-terminus is exposed to the analyte being passed over the sensor surface, promoting antigen-antibody binding. In the case of antibody-based immuno-sensors, the analyte may contain extracellular vesicles, bacteria or cell fragments, and QCM can serve as a rapid method for quantifying whether the target antigen is present in the analyte.
As well as exploiting the Biotin-Streptavidin interaction, high-frequency short-wavelength UV pulses can be used to orientate antibodies onto a gold surface (such as that found in QCM sensors). This UV energy disrupts the disulphide bond present at the C terminus of the antibody, increasing the favourability of the C terminus-Gold binding. This leads to an improvement in antibody orientation and, ultimately, an improvement in the selectivity and sensitivity of the QCM biosensor.
Quartz Crystal Microbalance future uses
QCM has vast potential as a high-throughput biological assay of the future. QCM's ease of use, selectivity and sensitivity could allow for bedside diagnosis and analysis of a wide variety of conditions affecting the patient of tomorrow. As well as its use in clinics, QCM is a technique that should be considered by labs worldwide. Its ability to be efficiently multiplexed for rapid biomarker validation and sample analysis cannot be overstated, and the relatively low set-up cost makes it a highly attractive prospect.
From biomarker discovery through to clinical application, QCM can be used every step of the way. With proper chip production, the end product can be used by someone with little to no technical knowledge, something that cannot be said for other techniques such as WB or FC.
The next generation of highly quantitative and sensitive techniques emerges as research shifts away from conventional, semi-quantitative methods such as WB and IF. These techniques, such as QCM and SPR, aim to drastically improve the selectivity and sensitivity of assays, all while accelerating workflows. While QCM has been used extensively in chemistry, its use in biological research still requires further validation and exploration, and it is fascinating to see where this technology can lead!
Bibliography
[1] S. Bruckenstein and M. Shay, ‘Experimental aspects of use of the quartz crystal microbalance in solution’, Electrochimica Acta, vol. 30, no. 10, pp. 1295–1300, Oct. 1985, doi: 10.1016/0013-4686(85)85005-2.
[2] A. Golczewski, A. Kuzucan, K. Schmid, J. Roth, M. Schmid, and F. Aumayr, ‘Ion-induced erosion of tungsten surfaces studied by a sensitive quartz-crystal-microbalance technique’, Journal of Nuclear Materials, vol. 390–391, pp. 1102–1105, Jun. 2009, doi: 10.1016/j.jnucmat.2009.01.279.
[3] A. Wajid, ‘On the accuracy of the quartz-crystal microbalance (QCM) in thin-film depositions’, Sensors and Actuators A: Physical, vol. 63, no. 1, pp. 41–46, Sep. 1997, doi: 10.1016/S0924-4247(97)80427-X.



