Replication Race: Scientists unravel why Omicron spreads faster
Written by Tiago Marcos Ph.D, guest author from the University of Edinburgh.
Hui et al. (2022, accelerated peer review) investigate how Omicron replicates faster than other SARS-CoV-2 variants in the human upper airways versus, and Omicron's reliance on TMPRSS2 dependent and independent cell entry mechanisms.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects the lungs, rendering respiratory distress and the associated hospitalization, ICU and death outcomes. SARS-CoV-2 has mutated into a multitude of variants, with the virus showing a considerable amount of evolutionarily plasticity. Following World Health Organization briefings, several variants were classified by health institutions and governments around the world as variants of concern. The Omicron variant was the latest of such variants. Many of the new variants partly escape vaccine and post-infection antibody immunity and it is therefore crucial to understand the biological processes that are altered by such gene sequence mutations.
Omicron's transmission rate is higher than previous variants and causing it to spread quickly during late 2021 and early 2022. It thought that the upper respiratory tracts of the bronchus and lungs may be affected differently by the virus variants. As such, Hui and colleagues (2022) set out to understand this by examining the replication rates of Omicron in the human bronchus and lungs. Ex vivo human tissue of bronchus (higher respiratory tract) and lung (lower respiratory tract) cultures were exposed to 50% tissue culture infectious dose (TCID50) titrations. Viral replication kinetics were evaluated after 24, 48, and 72 hours post infection for WT, Delta and Omicron variants. By comparing the original (WT) variant with the Delta and Omicron variants in bronchus tissue, a higher rate of replication was observed for Omicron after 24 and 48 hours post infection as compared to WT and Delta. At 72 hours post infection, both Omicron and Delta showed higher replication rates compared to WT (but the difference was not significant between Omicron and Delta). Conversely, in the lungs the ability of the Omicron variant to replicate was significantly reduced compared to WT at all three time points. Together, these results suggest that Omicron has a reduced replicative capability in the lung but increased replicative potential in the bronchus.
 |
|
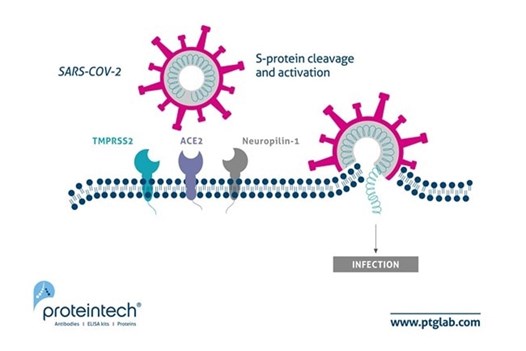
Figure 1. Updated schematic of SARS-CoV-2 host cell entry to include Neuropilin-1. Image originally adapted from Journal of Virology paper by Heurich et al. PMID: 24227843. |
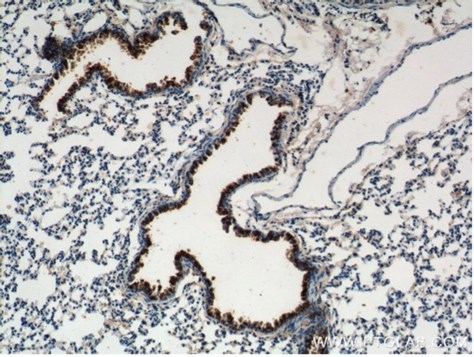
Bronchus cell type tropism of SARS-CoV-2 variants was assessed in three cell types: ciliated, goblet, and club cells (also known as Clara cells). Proteintech’s uteroglobin/club cell protein 10 (CC10) polyclonal antibody was used as a marker for club cells (10490-1-AP, 1:100, Figure 3 of the article). This allowed the research team to compare the expression of SARS-CoV-2 specifically in club cells and conclude that Omicron tropism is similar to WT and Delta, Beta and Alpha variants.
|
|
|
Figure 2. IHC staining of mouse lung using 10490-1-AP, Immunohistochemical analysis of paraffin-embedded mouse lung tissue slide using 10490-1-AP (Uteroglobin Antibody) at dilution of 1:200 (under 10x lens). |
Canonically, SARS-CoV-2 viral entry into cells is thought to be mediated by the host cell surface membrane ACE2 (angiotensin-converting enzyme 2) receptor in conjunction with other mediators such as neuropilin-1 and transmembrane protease serine 2 (TMPRSS2). Hui et al. also demonstrated a higher mRNA expression of ACE2 (long and short forms) in the bronchus than that in the lungs. Viruses can gain entry into the cell by fusing with the membrane or via endocytosis. Fusion is mediated by TMPRSS2, while other endocytic mechanisms require proteins such as cathepsin, a lysosomal protease. The Omicron spike protein has 15 receptor binding domain amino acid substitutions suggesting that cell entry mechanisms and subsequent replication rates may be different than previous variants. To test whether Omicron has a cell entry mechanism different to that of other variants, Vero E6/T2 cells were infected with viral variants in the presence of camostat mesylate (a serine-protease which targets TMPRSS2) versus control. Infection viability was partially reduced by camostat mesylate in WT, Omicron, and Delta variants, with Delta showing a larger effect. Further, a cathepsin inhibitor (E64d) presence greatly reduced viral viability on Omicron titrated cells to 5% of that observed in control cells. These results suggest a higher dependence of the Omicron variant on the endocytic cell entry pathway.
In a nutshell, the Omicron variant seems to replicate faster in the bronchus than in the lungs. This may justify its higher transmissibility as higher viral loads are present in the upper airways in Omicron infections. Omicron’s lower replication competence in lung also explains the milder respiratory symptoms seen in infected patients. As with many clinical studies, Hui and colleagues study is statistically low powered (making experiments with all variants in parallel difficult) as appropriate clinical subjects can be difficult to find, but it provides pilot data that gives important clues as to what clinical interventions may work best given that TMPRSS2 inhibitors may be less effective against the Omicron variant.
Related COVID-19 Entry Mechanism Antibodies
|
Cat number |
Product name |
Applications |
Species Reactivity |
|
10490-1-AP |
IHC, IF, FC |
Human, Mouse, Bovine |
|
|
21115-1-AP |
IHC, WB, IP |
Human, Mouse, Rat |
|
|
66699-1-Ig |
WB, IHC, IF, ELISA |
Human, Mouse |
|
|
80063-1-RR |
IHC, ELISA |
Human |
|
|
14437-1-AP |
IHC, WB, IP |
Human, Mouse, Rat |
|
|
60067-1-Ig |
WB, IF, FC, ELISA |
Human, Pig |
View more products for COVID-19 research here.
Related Content
Neuropilin-1 joins ACE2 as SARS-CoV-2 gateway
How Nanobodies could advance Sars-CoV diagnostics
Neutralizing antibodies and their role in COVID-19 research
How fluorescent proteins can be applied in SARS-CoV-2 research
Serious about Serology: Understanding COVID19 Antibody Tests
Characterization of COVID-19 antibodies by Bio-Layer Interferometry using Nano-CaptureLigands

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.