Flow Cytometry Multicolor Panel Design
リンク
- Introduction to flow cytometry
- Flow Cytometry Applications
- Flow Cytometry Fluorophores and Dyes
- Flow Cytometry Multicolor Panel Design
- Flow Cytometry Sample Preparation
- Protocol for studying extracellular and intracellular proteins
- Experimental protocol to study cell viability and apoptosis
- Popular antibodies for flow cytometry
Flow cytometry multicolor panel design
Many flow cytometry experiments use more than one fluorophore to stain samples. Multicolor experiments are a powerful tool that not only enable cost savings by analyzing fewer samples at a time but also allow for the gathering of more information using single cell analysis. They also make it possible to examine relationships between targets – e.g, examining whether cells positive for one target also express the other one.
Filters in flow cytometers
Every fluorophore has characteristic excitation and emission spectra. The wavelength of the laser light is chosen based on its excitation spectrum. Prior to detection, the emitted light is filtered through sets of optical filters. There are three types of filters:
-
Longpass (LP) – filter light above a certain wavelength,
-
Bandpass (BP) – filter light between certain wavelengths,
-
Shortpass (SP) – filter light below a certain wavelength.
It is easy to choose appropriate filters in single fluorophore experiments. However, in multicolor experiments with fluorophores excited by the same laser, sets of filters need to be chosen with caution. The example setup depicted in Figure 3 shows the emission spectra of three fluorophores. SP filter 1 filters light below 520 nm, which allows it to gather the spectrum of fluorophore 1. BP filter 2 (600/15 nm) collects light from fluorophore 2, while LP filter 3 (630 nm) collects most of the spectrum of fluorophore 3. Using appropriate filter sets, the user is able to efficiently collect signals originating from different fluorophores.
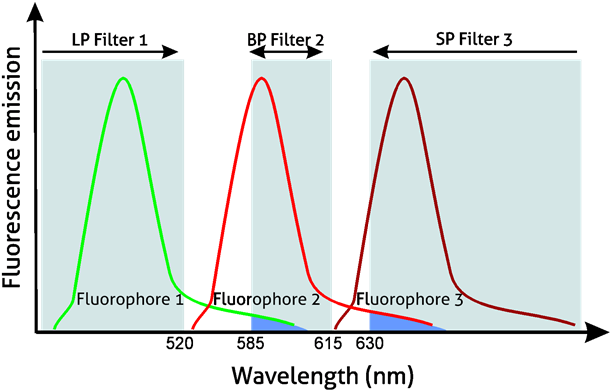
Figure 3. Emission spectra of three fluorophores – considerations for light filtering and compensation using LP, BP, and SP filters.
Compensation in flow cytometry
However, the emission spectra of different fluorophores often overlap (Figure 3 – marked in blue), which means that additional fluorescence compensation is needed. In the example given, filters 2 and 3 filter some of the light emanating from fluorophores 1 and 2 respectively. Therefore, in order to determine which cells in the analysis are positive or negative for a given fluorophore, compensation is required. There are two types of compensation controls: 1) single stained controls and 2) fluorescence minus one (FMO) controls. Compensation can be computationally applied after running all samples as long as the two controls are also included.
-
Single stained controls are cell samples or beads that are stained with only one fluorophore. Cells are good for compensation provided that they are a mix of both positive and negative cells for a certain fluorophore reagent. This way it is possible to distinguish between positive and negative events for a single channel. Commercially available compensation beads are an alternative to single stained cell controls. They are composed of a mix of beads, where a proportion of them have binding sites for antibodies. They often provide a better distinction of negative and positive events and permit the saving of scarce cell sample to set up controls, but are not compatible with all antibody species.
-
In experiments using more than two fluorophores, it is vital to also include FMO controls, which are samples stained with all used fluorophores in the experiment apart from one. They are important for assessing fluorescence spreading and for final gating.
Voltage settings are important for the correct interpretation of results. Voltages are set so that FSC and SSC settings are able to exclude cell debris. Actual settings depend on the experimental setup and should first be adjusted with single stained control samples to make sure that all events are within the scale.
Controls in flow cytometry
Irrespective of whether the experiment is multicolor or not, there are additional controls that should be included in all flow cytometry experiments – these are summarized in Table 2.
Table 2. Controls in flow cytometry experiments.
| Control | Reason to include |
| Unstained sample | To account for cell autofluorescence |
| Single stained control | To account for spectral overlap |
| Isotype control | To account for non-specific binding with a species-matched antibody |
| Fluorescence minus one (FMO) control | To adjust for spectral overlap in multicolor experiments |
| Positive control | To ensure that the staining procedure is successful |
| FC blocking control | To block Fc receptors in immune cells – essential in staining B cells, dendritic cells, monocytes and macrophages |


