Choosing The Right Western Blot Detection Method
リンク
- Western Blot Protocol
- How To Optimize Your Western Blot
- SDS-PAGE Gel Recipes
- How To Optimize Your Results With Low MW Proteins
- Tricine Gel Recipe For Low Molecular Weight Proteins
- Choosing The Right Lysis Buffer
- Choosing The Right Western Blot Detection Method
- Western Blot Troubleshooting: Why Does The Observed Protein Molecular Weight (MW) Differ From The Calculated One?
- Western Blot Troubleshooting: High Background
- Western Blot Troubleshooting: Weak/No Signal & Other
- Western Blot ppt
- Western Blot Video Protocol
Introduction
The Western blot is a widely used technique that has been around for over 40 years. Proteins are separated by size through a gel by electrophoresis, from where they can be transferred to a membrane and then identified using primary and subsequently secondary antibodies. On your membrane, these antibodies should produce a band specific to the molecular weight, which can be understood using the protein ladder run in conjunction with your samples in the gel. Over the years, the general technique has remained very similar, although the detection methods have been expanded and refined to allow for more precise and quantitative analysis of proteins.
The method you choose will depend on your experiment and the availability of equipment in your lab. Understanding the different options, how they work, and what the benefits are may make it that much easier to identify your protein and contribute to the success of your experiment.
Radioactive detection
Historically, antibodies for Western blot were tagged with radioactive labels. This radioactive detection method began declining in popularity as other methods were developed, owing to the hazards associated with exposing scientists to radiation.
- A band of protein is detected by exposing an X-ray film to the membrane, often for up to 48 hours.
- The film develops a dark band with exposure to the radioactive tag relative to the weight and amount of protein present.
| Advantages | Sensitive |
| Disadvantages | Health and safety risks, expensive, time-consuming |
| Top tip | Avoid! |
Enzymatic detection
Colorimetric and Chemiluminescent
This method uses secondary antibodies that are conjugated to enzymes and therefore require a substrate to work. These antibodies are either horseradish peroxidase (HRP) or alkaline phosphatase (AP) and can be used in either colorimetric or chemiluminescent detection methods.
The images produced can be analyzed quantitatively by densitometry (intensity of signal) or by spectrophotometry. A proper loading control must be used in the experiment if you want to be able to conduct any quantitative analysis using densitometry methods.
Colorimetric
In colorimetric detections, the enzyme attached to the secondary antibody triggers a reaction with a substrate to produce a colored precipitate.
- Add appropriate substrate to membrane probed with antibodies
- Insoluble precipitate accumulates on and stains the membrane, visible to the eye
- Stop development by washing the membrane, judged by your own approximation.
HRP in colorimetric detection is very cost-effective but may fade on exposure to light and can produce non-specific staining. By contrast, AP in colorimetric detection produces a stable substrate that will not fade and can also use different substrates to produce different colors on the same membrane.
The main limitation of colorimetric detection lies in its sensitivity, with protein in the nanogram range required to produce a signal. This is far more protein than is necessary in other methods, which can detect a signal in the femtogram range.
| Advantage | Fast, cheap, visual, no specialist equipment required |
| Disadvantages | Not very sensitive, lots of protein required. |
| Top tips |
|
Chemiluminescent
In chemiluminescent detections, the enzyme attached to the secondary antibody triggers a reaction with a luminescent substrate that produces light as a by-product. As with colorimetric detection, the HRP and AP enzymes are used with appropriate substrates.
- Add the substrate to membrane, with the former available in various strengths and stabilities that may be optimal for your experiment
- Detect the resulting light signal by exposure to X-ray film or charge-coupled device (CCD) imaging
- Follow with quantitative analysis, which is highly dependent on the final image quality.
The film detection method is widely considered to be the most sensitive, but you must work quickly while the enzymatic reaction is still stable on the membrane. CCD imaging is very useful because it is simple and automated, but for very low expressed proteins it may be safest to use film exposure.
Due to potential cross-reactivity on the membrane and there being no way to distinguish bands of similar sizes, multiplexing different primary antibodies is difficult. Although the membrane can be stripped and re-probed, this can reduce the signal and affect results.
| Advantages | High sensitivity, best for low expressed proteins |
| Disadvantages | Requires specialist equipment, multiplexing difficult. |
| Top tips |
|
Fluorescent detection
Fluorescent detection uses secondary antibodies that are conjugated to specific fluorophores , so no additional substrate is necessary, though a specially designed digital imaging system is needed.
- Fluorophores are excited by infrared, LED, or visible light using this system
- Image collected and quantified.
The fluorophores themselves produce a signal that is far more stable than the enzymatic detection method, lasting for months or even years. The fluorescent signal also has a much greater dynamic range than chemiluminescence, so there is better linearity in the detection limits. However, fluorescence detection is generally considered to be less sensitive than enzymatic detection.
Different fluorophore-tagged antibodies with different emission wavelengths can be used on a single Western blot membrane, which means that multiplexing your experiment is possible without stripping and re-probing the membrane, so there is no risk of reducing the signal.
| Advantages | Stable, multiplexing straightforward |
| Disadvantages |
Less sensitive, requires specialist equipment. |
| Top tips |
|
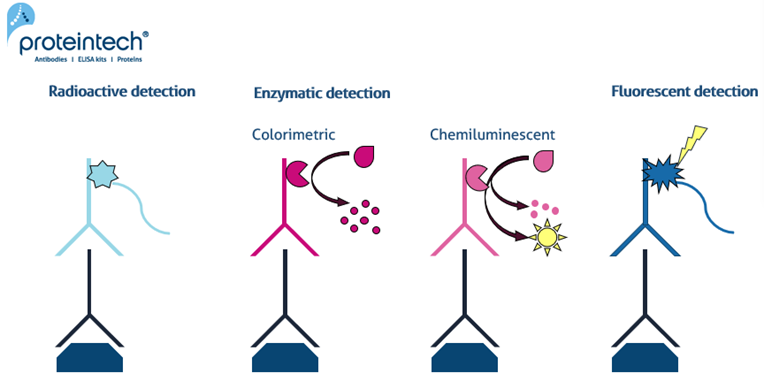 |
| Figure 1. Different detection methods for Western Blot including radioactive, enzymatic (colorimetric & chemiluminescent) and fluorescent analysis. |
Final remarks
Whichever detection method you choose, the resulting image will ultimately depend on the overall design of your experiment, the use of proper controls, and the sensitivity of your antibody, as well as other optimization steps in your protocol.
The decision on which detection method to use may be made solely for you, based on your lab, or you may have the ability to trial a few options – personal preference, as well as scientific reasons, can sometimes influence a particular choice.
|
|
Sensitivity | Multiplexing | Enzyme | Substrate | Stability |
| Enzymatic: Colorimetric |
Low |
Possible |
AP advised |
Various |
Hours |
| Enzymatic: Chemiluminescent |
Highest |
Only with re-probing |
HRP advised |
Luminol based |
Hours |
| Fluorescent |
Low |
Easy |
None |
None |
Months - years |


