タンパク質のリン酸化:ON/OFFどちらかの制御にとどまらない複雑な仕組み
Joris Frenz著(ドイツ癌研究センター、博士研究員、Proteomics and Cancer Cell Signaling研究室、ドイツ/ハイデルベルク)
リン酸化を介したタンパク質制御
細胞機能を制御する主力分子であるタンパク質は、その制御に驚くべき複雑性を発揮します。タンパク質の特徴を定義づける直線的なアミノ酸配列の多様性に加え、刻一刻と変化する翻訳後修飾がタンパク質の機能を複雑化させています。一連の翻訳後修飾の中でも、タンパク質のリン酸化はタンパク質の活性状態と不活性状態を切り替える分子スイッチとして機能し、細胞機能の制御機構の中枢的な役割を果たします。
プロテインテックの関連製品:リン酸化タンパク質検出用抗体
多くの場合、タンパク質内の単一のアミノ酸残基のリン酸化は、タンパク質の活性化/不活性化状態を切り替える役割を果たすと考えられます。その一方で、多くのタンパク質は複数のアミノ酸残基のリン酸化イベントによって、リン酸化のパターンに依存した複雑な制御ネットワークを形成します。このようなタンパク質リン酸化の動的かつ状況依存的な性質が、転写、細胞周期の進行、シグナル伝達等の極めて重要なプロセスを規定する分子イベントを統合し、細胞応答を形成します。翻訳後修飾という複雑なプロセスにおいて、タンパク質内の複数のアミノ酸残基のリン酸化による相乗効果や拮抗作用は、細胞生理や病態を決定する制御機構に関与します。
本稿では、複数のリン酸化部位を有する特定のタンパク質に注目し、単一部位のリン酸化によるON/OFF制御よりも、高度で精緻な制御機構について解説します。
転写因子p53は、最も研究されているがん抑制タンパク質の一種で、『ゲノムの守護神』として知られています。ゲノム安定性を維持する以外にも、細胞周期の進行、DNA修復、アポトーシスにおいて極めて重要な役割を果たします。p53機能の調節不全は多岐にわたるヒトのがんに関与しており、細胞の完全性の維持や異常な細胞増殖の阻止におけるp53の重要性を強く示しています。
p53の働きは細胞にとって本質的に重要であり、p53は複数のリン酸化部位によって厳密に制御されています。p53のSer15残基がリン酸化されると、p53のDNA結合ドメインやC末端ドメインに存在するリシン残基のアセチル化が促進されます。また、C末端ドメインのリシン残基がアセチル化されると、MDM2(E3ユビキチンリガーゼ)によるp53のユビキチン化とそれに続くプロテアソームによるタンパク質分解が阻害されます。こうした仕組みによって、Ser15残基のリン酸化はp53を安定化します[1]。さらに、Ser15残基のリン酸化はSer9・Ser20・Ser46・Thr18残基のリン酸化も引き起こします。
また、Ser20・Thr18残基のリン酸化はMDM2からp53が遊離する際にも重要であることが判明し[2]、p53のリン酸化部位がそれぞれ異なるリン酸化を受けることによってp53の安定化と分解が厳密に制御されることが示されました。
p53の働きは、他の残基のリン酸化によっても、それぞれが異なる機構の制御を受けます。例えば、Ser46残基のリン酸化はアポトーシス促進因子として作用し[3]、Ser392残基のリン酸化はp53のミトコンドリアへの移行に影響を及ぼし、転写とは独立した機構によってアポトーシス誘発因子として働きます[4]。
プロテインテックの関連製品:p53関連製品
|
Phospho-P53 (Ser15)ポリクローナル抗体(カタログ番号:28961-1-AP) |
 |
|
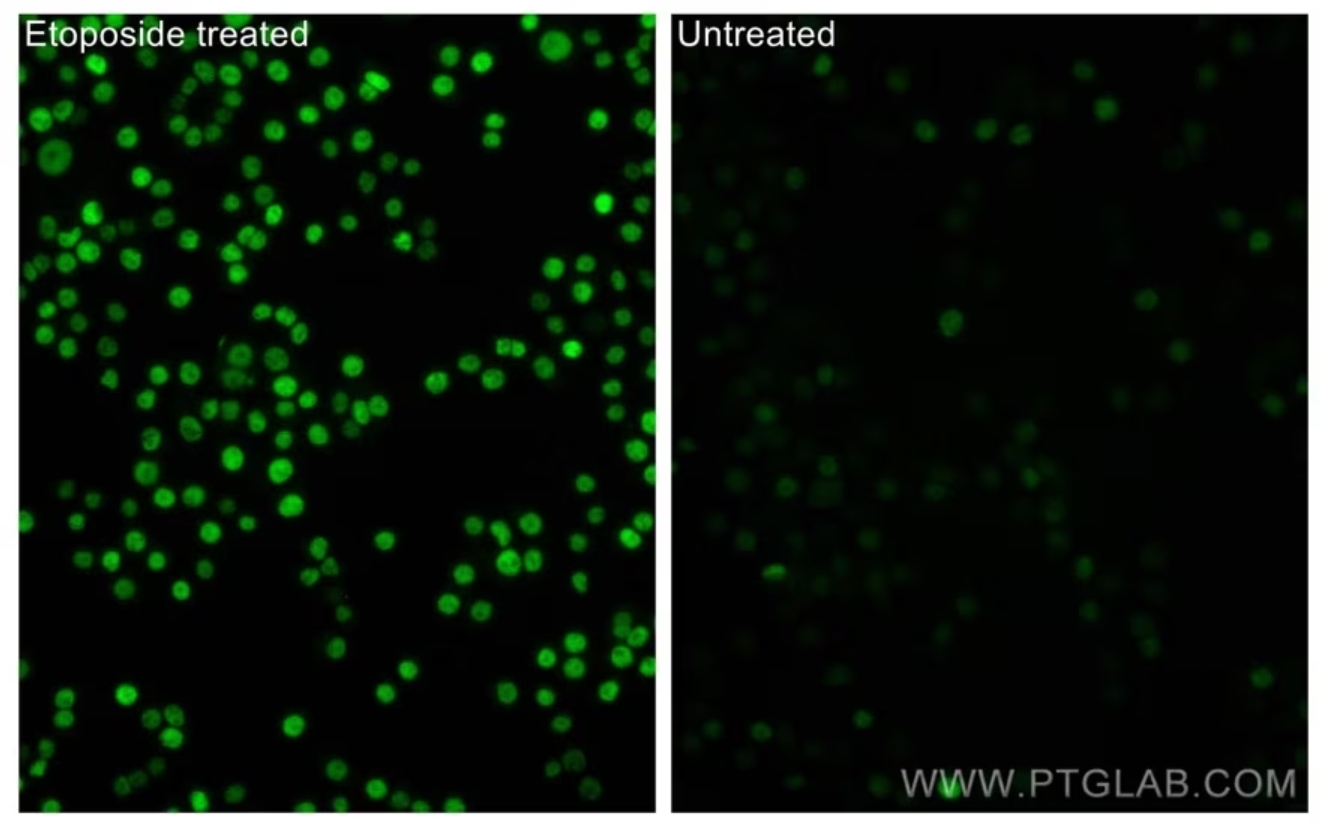
Phospho-P53(Ser15)抗体(カタログ番号:28961-1-AP、希釈倍率1:100)/CoraLite®488-Conjugated AffiniPure Goat Anti-Rabbit IgG(H+L)抗体を使用したHT-29細胞(4% PFA固定)の免疫蛍光染色。左:エトポシド処理。右:無処理。 |
サイクリン依存性キナーゼ1(CDK1:Cyclin-dependent kinase 1)は、G2期から有糸分裂への移行を調整する、細胞周期の極めて重要な制御因子です。CDK1はその他のサイクリンと連携して、細胞分裂で重要な役割を果たす複合体を形成し、染色体分離や細胞増殖の適切な進行を司ります。CDK1の働きは細胞周期の進行を正確に維持するために厳密な制御を受け、ゲノム安定性の維持や細胞増殖において極めて重要な役割を担っています。CDK1の調節不全は様々な疾患と関連しますが、その中でも特にがんと深く関係します。
サイクリンH-CDK7複合体によるThr161残基のリン酸化はCDK1の活性化に必須のイベントで、CDK1が活性化されることによって細胞周期の進行やその他の複雑なシグナル伝達ネットワークが駆動されます。同時に、Wee1とMyt1によるCDK1のTyr15・Thr14残基のリン酸化はCDK1活性を抑制し、適切な時期よりも早い段階で有糸分裂が開始されるのを防ぎます。Cdc25ホスファターゼは、これらのタンパク質活性を抑制するリン酸化とは逆の作用を示し、CDK1のTyr15・Thr14残基を脱リン酸化することでCDK1の活性化を促進します[5]。
リン酸化による活性化(Thr161)と抑制状態(Tyr15・Thr14)の均衡が、CDK1が活性型として機能するか不活性化状態にとどまるかを決定づけます。キナーゼ活性とホスファターゼ活性の時間的な協調により、CDK1の働きは細胞周期の進行過程と厳密に連動し、G2/Mチェックポイント未通過のまま有糸分裂が開始されるのを防ぎます。CDK1等が関与する複雑な制御機構は、単純に活性化/不活性化状態の切替スイッチとしての役割を果たすだけでなく、細胞内部や外部のシグナルに対する緻密な細胞応答を可能にします。
プロテインテックの関連製品:CDK1関連製品
タウ(Tau)タンパク質は、神経細胞の構造や機能の維持に極めて重要な微小管関連タンパク質です。正常な状態では、タウタンパク質は微小管を安定化し、円滑な細胞内輸送に寄与しています。アルツハイマー病(Alzheimer's disease)等の神経変性疾患では、タウタンパク質の異常なリン酸化によるタウタンパク質機能の破綻が神経原線維変化の原因となって神経変性が生じます。微小管動態におけるタウタンパク質の役割の解明は、神経変性疾患の病態の解明や効果的な治療介入法を開発するにあたり欠かすことのできない研究です。
微小管へのタウタンパク質の結合制御は、タウタンパク質のリン酸化状態に大きく依存します。タウタンパク質はC末端側の微小管結合ドメインを介して微小管と相互作用します。具体的には、微小管結合リピートに相当するSer262・Ser356残基のリン酸化によってタウ‐微小管相互作用が弱まり、条件によってはタウタンパク質の凝集性が低下するとされます。これに加え、微小管結合ドメインの外側のアミノ酸残基(例:Ser214・Thr231)のリン酸化によって、タウタンパク質の微小管に対する親和性が低下します。
こうした知見は、タウタンパク質内の異なるアミノ酸残基のリン酸化が、タウタンパク質の凝集に対照的な影響を及ぼし得ることを示唆しています。また、ペプチジルプロリルイソメラーゼであるPin1とリン酸化Thr231残基間の相互作用によりタウタンパク質内のペプチド構造はシス(cis)型からトランス(trans)型に構造変化し、ホスファターゼであるPP2Aとタウタンパク質が結合しやすくなって過剰なリン酸化が解消され、タウタンパク質と微小管の結合能が回復します[6]。
プロテインテックの関連製品:Tau関連製品
RNAポリメラーゼⅡ(RNA Pol II:RNA Polymerase II)は、遺伝子発現の核心的な酵素であり、DNA配列を鋳型にしてmRNAを合成(転写)します。mRNA生合成の主要なメディエーターとして、RNA Pol IIは転写開始・伸長・終結の複雑なプロセスを統制します。多種多様なタンパク質を生合成するには、RNA Pol IIによるmRNA合成の各プロセスが正確に制御される必要があります。そのため、RNA Pol IIは細胞内プロセスで中核的な役割を担い、真核生物における遺伝子発現を根底で支える存在といえます。
RNA Pol IIのSer5残基のリン酸化は、mRNAの合成開始時や、プロモータークリアランス促進時、キャッピング酵素が動員される際にmRNAの安定性を保つうえで極めて重要な役割を果たします。転写が開始すると、サイクリン依存性キナーゼCDK9(Cyclin-dependent kinase 9)と調節サブユニットであるサイクリン(Cyclin T)で構成される転写伸長因子P-TEFb(positive transcription elongation factor b)が動員されるまで、RNA Pol IIは転写開始部位の約25~50 bp下流で停止します。P-TEFbはRNA Pol IIをリン酸化し、転写伸長を促進します。Ser5残基とSer2残基のリン酸化は連続的かつ動的であり、Ser5残基がリン酸化されることで、Ser2残基がP-TEFbによってリン酸化される条件が整います。その他に、RNA Pol IIのC末端ドメインのSer7残基にもリン酸化が生じます。このSer7残基のリン酸化はsnRNA(small nuclear RNA)の転写やインテグレーター複合体(Integrator complex)の動員に関係があるとされています[7]。
この組織化されたリン酸化カスケードによって、協調的かつ制御的な転写プロセスが確実に進行し、転写開始とRNA鎖伸長が紐付けられ、遺伝子発現とmRNA合成が緻密に制御されます。
プロテインテックの関連製品:RNA Polymerase II関連製品
Protein Kinase Bとしても知られるAKTは、細胞の生存維持・増殖・代謝において中心的な役割を果たすシグナル伝達タンパク質です。AKTは様々な受容体の下流で働き、タンパク質生合成・グルコース代謝・アポトーシス等の様々な細胞内プロセスを制御します。複雑なシグナル伝達網に関わることから、AKTは生理学的反応における極めて重要なメディエーターであり、がんや代謝性疾患のような疾患に伴う異常な細胞シグナル伝達を解明するにあたり重視すべき対象となっています。
シグナル伝達経路の開始に際し、AKTはPDK1(3-phosphoinositide-dependent protein kinase-1)によるThr308残基のリン酸化を受けます。この過程では、AKTがPIP3(Phosphatidylinositol-3,4,5-triphosphate)に結合する必要があり、結合によってAKTのコンホメーション変化が誘導され、細胞膜上に存在するPDK1に対してAKTのキナーゼドメインが露出します。AKTが最大限に作用を発揮するには、mTORC2(mechanistic/mammalian target of rapamycin complex 2)によってSer473残基がリン酸化される必要があり、このリン酸化によってAKTの活性は顕著に増大します。それとともに、Thr308・Ser473残基のリン酸化は、増殖因子に応答してAKTの活性が亢進していることを示すマーカーの役割を果たします。リン酸化AKTは、FoxOファミリーに属するタンパク質・GSK-3β・mTOR経路の上流因子をリン酸化する働きによって、下流の多様な生物応答を統合的に調節します[8]。
AKT活性化に伴う2通りのリン酸化の相互干渉によって、PI3K/AKT、mTOR等の様々な経路は統合され、AKT活性や、細胞増殖・生存維持等の下流の細胞応答が緻密に制御されます。
プロテインテックの関連製品:AKT関連製品
リン酸化だけでは語れない細胞プロセスの制御機構
本稿で紹介した事例は、リン酸化を介したタンパク質制御の複雑性を浮き彫りにし、タンパク質機能に対するリン酸化の相乗効果や拮抗作用によって、複数の相互作用が緻密に関与することを提示しています。こうした事例は、多種多様なシグナル伝達経路にまたがって認められ、解明が進んでいるタンパク質制御機構の複雑性を示しています。しかし、膨大な種類の細胞プロセスの存在を考慮すれば、その複雑性は想像以上に多層的であると考えられます。タンパク質のリン酸化が、リン酸化を介した制御機構の複雑な構成に寄与している例は本稿で触れた以外にも数多く認められます。注目すべき点は、様々な相互作用が複雑に関与するという点です。大抵はリン酸化による制御機構に加え、転写後調節の他、翻訳後修飾、制御タンパク質、補因子、その他の細胞内分子による相互作用が多岐に関与し、細胞制御やシグナル伝達の複雑性を高度化しています。
その他のタンパク質リン酸化による高度な制御機構
- 細胞のシグナル伝達には、ERK・JNK・p38等のMAPK(Mitogen-activated protein kinase)が関与します。
- MAPKは複数のリン酸化部位が関与するリン酸化カスケードを介して活性化され、細胞内での機能や応答が調節されます。
プロテインテックの関連製品:MAPK関連製品
GSK-3(Glycogen Synthase Kinase 3)
- GSK-3は恒常的に活性が高く、様々な細胞内プロセスに関与するセリン/スレオニンキナーゼです。
- GSK-3βのSer9残基の他、いくつかの部位のリン酸化状態に応じて、GSK-3の活性は多面的に制御されます。また、このリン酸化は上流に存在するその他のシグナル伝達経路の影響を受けます。
プロテインテックの関連製品:GSK3関連製品
AMPK(AMP-Activated Protein Kinase)
- AMPKは、細胞内のAMP/ATP比やADP/ATP比の変動を検知するヘテロ三量体キナーゼです。
- AMPKが活性化するにはThr172残基のリン酸化が必須であり、上流で働くLKB1・CaMKKβ等の複数のキナーゼがThr172残基のリン酸化に影響を及ぼします。
プロテインテックの関連製品:AMPK関連製品
NF-κB(Nuclear Factor-kappa B)経路
- NF-κBは免疫応答や炎症反応に関与し、刺激依存的に細胞質から核へ移行する転写因子です。
- NF-κBに結合する抑制性タンパク質であるIκBαは、Ser32・Ser36残基のリン酸化を受けます。この部位のリン酸化は、IκBαを分解するための目印となり、IκBαが分解されるとNF-κBは活性化できるようになります。
- NF-κBのp65サブユニットのSer536・Ser276残基のリン酸化は、転写活性の増強と核への局在に関係します。
プロテインテックの関連製品:NF-κB関連製品
事前の情報収集 ‐ タンパク質の背景情報を把握する
タンパク質の特定残基のリン酸化は、単純な活性化のON/OFF切替に還元できない場合があるという点を認識しておくことが重要です。そのため、研究開始前に必ず研究対象のタンパク質について徹底的に調査するよう留意してください。
研究を円滑に実施するうえで、以下のリソースが参考になります。
- UniProt(uniprot.org)
- PhosphoSitePlus(phosphosite.org)
- Phospho ELM(elm.eu.org)
- BioGRID(thebiogrid.org)
- PhosphoNET(phosphonet.ca)
プロテインテックは140品目を超えるリン酸化タンパク質検出用抗体を提供しています。
一連のリン酸化タンパク質抗体はこちらをご覧ください。
プロテインテックのパスウェイポスター
 |
 |
参考文献
1 - J Loughery, et al. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res. 2014 Jul;42(12):7666-80.
免疫沈降用アフィニティビース
<Nano-Trap 無料サンプル配布中!>
関連情報
リン酸化タンパク質をウェスタンブロット検出するためのヒントとコツ
翻訳後修飾(PTM:Post Translational Modification)とは?
サポート




