Nanobody(ナノボディ)とは?
アルパカ由来VHH抗体(Nanobody)を理解するための完全ガイド
Nanobodyの基本情報
Nanobody(ナノボディ)は、単一ドメイン抗体(sdAb:Single domain antibody)またはVHH(Variable heavy domain of heavy chain)とも呼ばれる、低分子(約15 kDa / 2 nm)の抗原結合ドメイン(可変領域)で、ラクダ科動物の免疫応答の一環として産生される重鎖抗体(HCAb:Heavy chain antibody、Heavy chain-only antibody)に由来します。研究にはアルパカがよく使用されますが、ラマ、ヒトコブラクダ、フタコブラクダもこの重鎖抗体を産生します。興味深いことに、重鎖抗体はサメからも発見されていますが、飼育や免疫化についてはアルパカの方が危険性は低いとされています。
マウス、ウサギ、ラット等のIgGのような従来型の抗体は、研究や医療分野において多くの用途に幅広く利用されています。この従来型IgGは2本の軽鎖と2本の重鎖で構成されるヘテロ四量体の複合体です。従来型抗体の抗原結合部位は、抗体のFab領域の軽鎖上部と重鎖上部の2か所の可変領域が相互作用する必要があります。免疫応答の一環として、ラクダ科動物は従来型のIgG1抗体に加え、重鎖抗体(HCAbサブクラス:IgG2・IgG3)も産生します。IgG1とは異なり、重鎖抗体は重鎖のCH1ドメインと軽鎖が欠損しています。したがって、重鎖抗体は抗原認識を1か所の可変領域のみで行います(図1)。
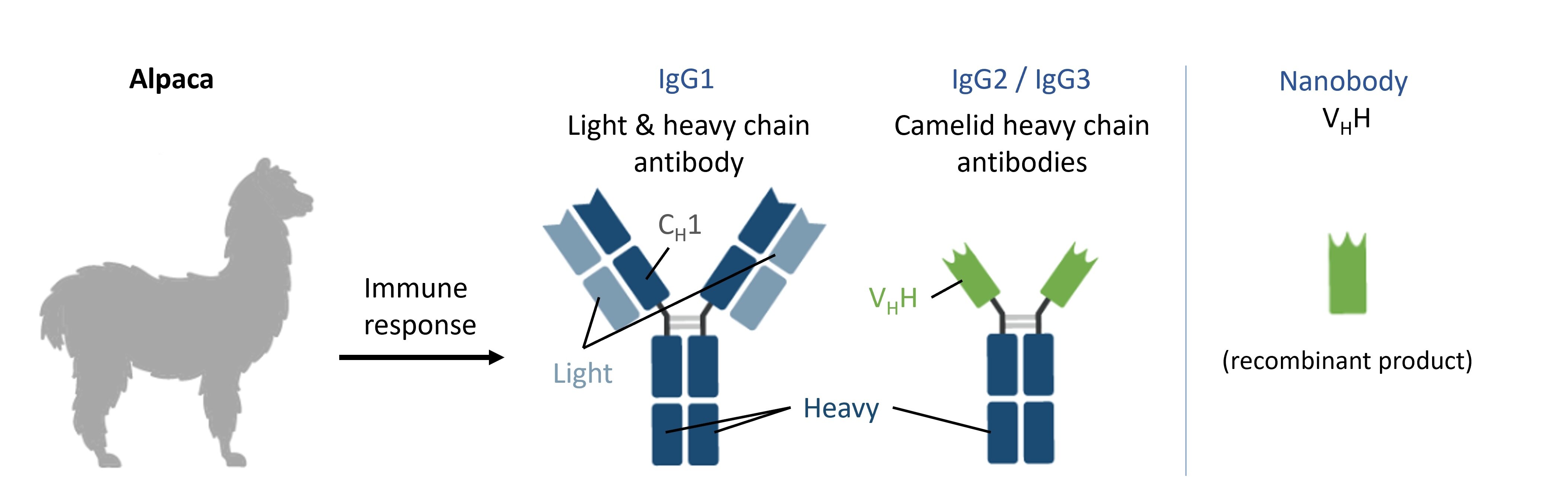
図1. アルパカの免疫応答と産生抗体の概要:アルパカの免疫応答では3種類のIgGサブクラス抗体が産生されます。IgG1抗体には重鎖と軽鎖が存在します。IgG2・IgG3サブクラス抗体は、重鎖のCH1ドメインと軽鎖が欠損した重鎖抗体です。重鎖抗体の可変領域はVHHと呼ばれ、VHHの組換え体はNanobodyとも呼ばれます。
抗原結合部位の複雑性が低減することによって効率的な組換え抗体の作製が可能になり、抗体メーカーはアニマルフリーでロット間変動のないNanobodyを製造できるようになりました。さらに、Nanobodyは低分子かつコンパクトな構造を有するため、高い熱安定性を示し、厳しいバッファー条件に耐性を示し、迅速かつ容易に組織へ浸透します。
Nanobodyは、修飾や標識を施したり、様々な基材へ吸着させることで、免疫沈降、免疫蛍光染色、免疫組織化学、タンパク質精製、ウェスタンブロット、従来型抗体が利用されているほぼすべてのアプリケーションにいたるまで、多様なアッセイに使用することができます。
プロテインテックは、様々なアプリケーションに使用可能なクロモテック(2020年よりプロテインテックの一部になりました)のVHH抗体(Nanobody®)製品を多数取り揃えています。VHH抗体(Nanobody®)製品のご利用に興味がある方は、以下の表1またはプロテインテックのChromoTek VHH(Nanobody®)試薬(ptglab.co.jp)をご覧ください。
プロテインテックは製品のテクニカルサポートやカスタム対応も承っております。お気軽にお問い合わせください。
プロテインテックのVHH抗体(Nanobody®)製品
表1. VHH抗体(Nanobody®)製品と適用可能なアプリケーション
|
アプリケーション |
VHH抗体(Nanobody®)試薬 |
|
ビオチン標識抗体を用いたBLI・SPR・ELISA |
Note:プロテインテックとクロモテックは、様々なアプリケーションに特化した製品を提供しています。ご購入前に各製品ページをご覧いただき、どのアプリケーションに適用可能かご確認ください。VHH抗体(Nanobody®)製品の使用について、実験の注意点や使用方法を確認したい場合は、製品ページに掲載されている各製品の個別プロトコールをご覧ください。個別プロトコールには、実験を計画する際の参考情報やポイントも記載されています。是非こちらの情報をご利用ください。
Nanobodyの作製法
目的の抗原を認識するNanobodyを作製するには、始めにアルパカ等の若い成体ラクダ科動物を免疫します。上述した通り、動物の免疫応答の一環として、ターゲットとなる抗原に対する重鎖抗体が産生されます。免疫したアルパカから少量の血液を採取し、その中からB細胞を単離します。得られたB細胞を使用して、組換え技術によってNanobodyを作製します。
Note:他の抗体メーカーが抗体の作製に短鎖ペプチドやペプチドプールを使用することが多いのとは対照的に、プロテインテックとクロモテックは大型のタンパク質断片や全長タンパク質を免疫原として使用します。その結果、タンパク質本来の三次元構造を示すエピトープに対して特異性の高い抗体やVHH抗体(Nanobody®)が得られます。
続いて、Multi-step nested PCRを実施し、B細胞cDNAプールのVHH配列を増幅します。適切な酵素によって制限酵素処理した後、VHH配列を発現ベクターにライゲーションし、大腸菌形質転換に使用します。
こうして、Nanobody(VHH抗体)免疫ライブラリーが完成します。
ターゲット抗原に対して親和性の高いモノクローナルNanobodyは、ファージディスプレイ法によるスクリーニングと評価を実施して同定・単離します。候補となるベクター配列を解析して高い親和性で結合するNanobody候補配列を明らかにし、大腸菌(E. coli)に発現させて精製します。
(Nanobody抗体の作製プロセスの詳細な解説の他、ファージディスプレイによるクローンセレクション、ナイーブVHHライブラリーについては、参考文献をご覧ください)
Nanobody(VHH)の構造
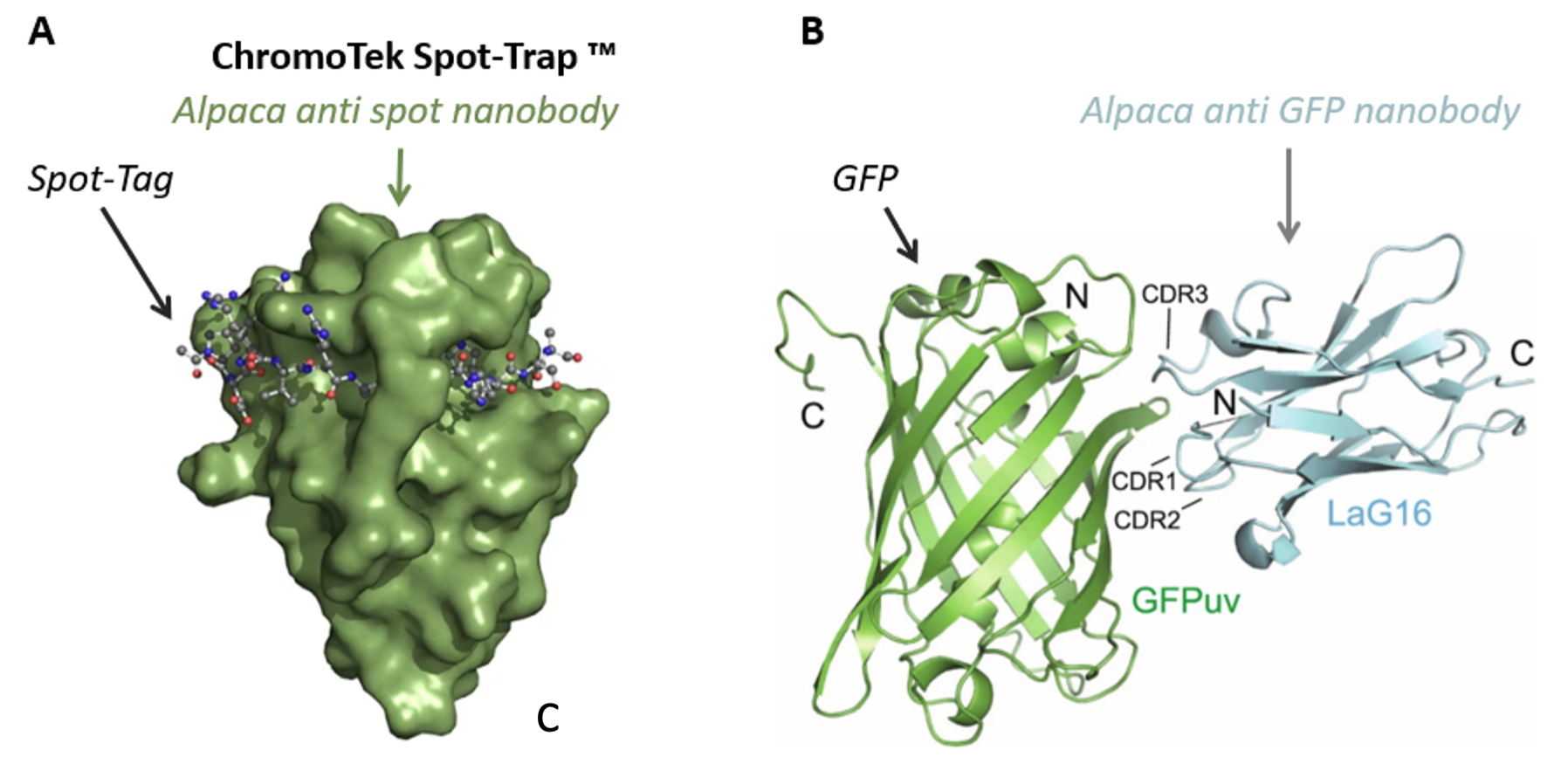
図2. Nanobodyと抗原の結合様式 A)クロモテックの抗Spot VHH抗体(Nanobody®)とSpot-Tag®(不活性な12アミノ酸残基(配列:PDRVRAVSHWSS)からなるペプチドタグ)の結合様式を示しています。タグが抗体上部を取り囲み、抗Spot VHH抗体(Nanobody®)のβシートと一体化したような構造をとります。Spot-Tag®ペプチドは抗体のアミノ酸残基の側鎖によって抗体に固定(クランプ)され、高い親和性で抗体と結合します(製品情報:Spot-Tag® 捕捉/検出システム)。B)3か所のCDR(相補性決定領域)が関与する抗GFP Nanobody LaG16の結合様式(Zhang et al. 2020)。
プロテインテックのVHH抗体(Nanobody®)は重鎖抗体の可変領域です。そのコンパクトな構造は、VHH抗体(Nanobody®)の「側面」を形成するβシートの積み重なり構造で構成されます。上部に存在する3つの相補性決定領域(CDR1/CDR2/CDR3)(CDR:Complementary determining region)は、VHHの抗原結合部位の柔軟な構造を決定づけます(図2)。CDRのアミノ酸鎖長とアミノ酸配列は可変的であるため、多様なターゲットエピトープと特異的に結合することが可能になります。
VHHが認識するエピトープの構造の違いに応じて、3か所のCDRドメインの異なるアミノ酸残基が結合に関与します。CDRとエピトープ間の相互作用は、抗原結合部位の全体的なコンホメーションや各CDRの相対的な位置関係に影響を及ぼします。
図2には、CDRが作用する結合様式を2種類の異なる表示方法で示しています。クロモテックのSpot-Trap®の場合、Spot-Tag®ペプチドはVHH抗体(Nanobody®)の上部を取り囲むことで、抗Spot VHH抗体(Nanobody®)のβシートと整列した状態になります。そして、抗Spot VHH抗体(Nanobody®)と結合したタグペプチドは固定(クランプ)機構によって抗体に埋没することで、極めて高い結合親和性を示します(図2A)。GFPuv(特定の波長で強い蛍光を発するGFP変異体)と抗GFP Nanobody LaG16との結合は、GFPのβバレル側面と接触する抗体の3か所のCDR領域すべてが連携することで維持されます(図2B)。
NanobodyのC末端は、重鎖抗体の全体構造の中でもCDRとは別の領域に面しているため、抗体と抗原の結合に関与しません。多くの場合、このC末端領域は、色素標識、担体との結合、別のタンパク質の融合に利用されます。
プロテインテックのVHH抗体(Nanobody®)と従来型抗体の比較
VHH抗体(Nanobody®)の分子量
プロテインテックのVHH抗体(Nanobody®)の分子量は約15 kDaで、より迅速かつ容易に組織サンプル・臓器・実験動物へ透過するその特性は、免疫蛍光染色や免疫組織化学といったアプリケーションで極めて有利に働きます。また、エピトープ‐蛍光色素間の距離が短く(図3)高解像度解析が可能なため、超解像顕微鏡や共焦点顕微鏡等を使用するアプリケーションに特に適しています。さらに、VHH抗体(Nanobody®)は、VHH抗体(Nanobody®)よりも10倍以上大きい従来型の抗体ではアクセスできない特定のエピトープと結合できる場合があり、特に核のような細胞内の混雑環境下においても、より均一にサンプルを染色することができます。
プロテインテックの関連アプリケーション:免疫蛍光染色(IF)
免疫組織化学(IHC)
超解像顕微鏡観察
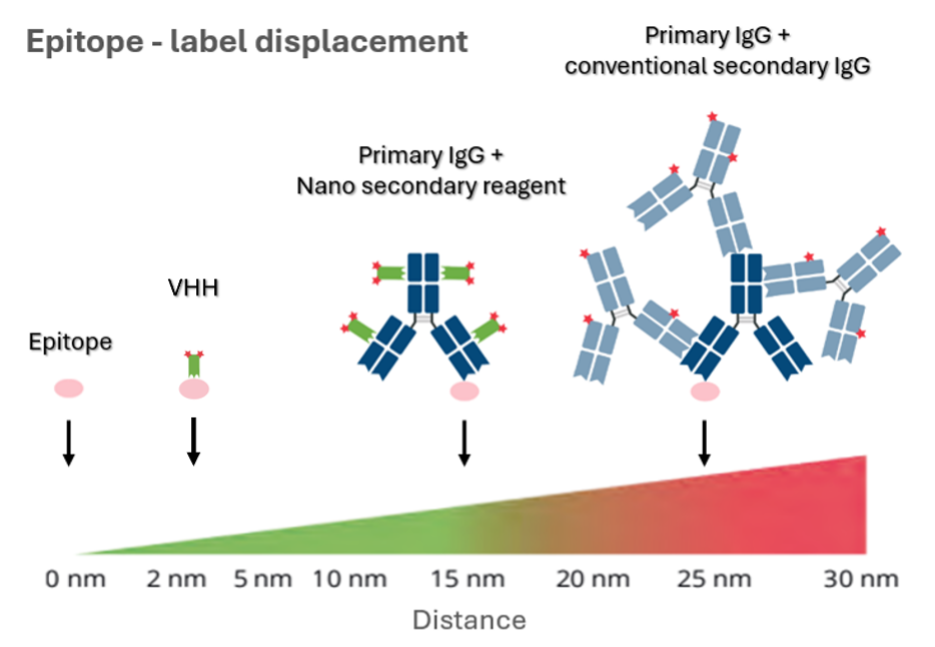
図3. (左→右):直接標識VHH抗体(Nanobody®)のエピトープ‐蛍光色素間の距離(約2 nm)は、Nano-Secondary®試薬で標識した一次抗体(最大15 nm)や従来型二次抗体で標識した一次抗体(最大30 nm)と比較して最も短くなります。プロテインテックのVHH抗体(Nanobody®)を採用することで、イメージングアプリケーションの解像度を高めることができます。緑:蛍光色素標識VHH抗体(Nanobody®)、青:従来型一次抗体(IgG)、水色:従来型蛍光色素標識二次抗体(IgG)(赤:蛍光色素、ピンク:エピトープ)
プロテインテックのVHH抗体(Nanobody®)の製造・大腸菌発現・再現性
プロテインテックのVHH抗体(Nanobody®)を作製する際は、ラクダ科動物を免疫してVHH免疫ライブラリーを作製する最初の工程だけに動物が関与します。供した生体は殺処分せず、少量の血液を採取してB細胞を単離し、免疫ライブラリーを作製します。以降の工程は、そのすべてを完全なアニマルフリーで実施します。VHH抗体(Nanobody®)の分子量は小さく複雑なタンパク質フォールディングの過程を辿らないため、大腸菌(E. coli)による発現と精製をより短時間かつ低予算で実施することが可能となり、ロット間変動の懸念も払しょくします。
安定性
プロテインテックのVHH抗体(Nanobody®)は、構造変化しやすい要因が少なく非常に凝集した構造をとります。そのため熱安定性に優れ、長期間保存可能で、厳しい組成(例:~2 M NaCl、pH 4.0~10.0のバッファー、4 M Urea)のバッファーにも耐性を示します。さらに、熱安定性が高く、コンパクトな構造をとり、ターゲットに対する親和性が高いため、X線結晶構造解析におけるシャペロンとして利用される等、タンパク質の構造決定を実施するにあたり有用なツールであることが判明しています。
プロテインテックのVHH抗体(Nanobody®)を使用した免疫沈降とタンパク質精製
プロテインテックのVHH抗体(Nanobody®)は、あらゆるタイプの免疫沈降(IP・CoIP・ChIP・RIP)に使用できるだけでなく、アフィニティ精製にも使用することができます。従来型抗体と比較して、VHH抗体(Nanobody®)にはいくつかの利点があります。最も重要な点はVHH抗体(Nanobody®)の極めて高い特異性です。VHH抗体(Nanobody®)は特異性が高いため、バックグラウンドが極めて低く、非特異的な結合のない実験結果を得られます。さらに、図4に示す通り、VHH抗体(Nanobody®)には抗体重鎖や軽鎖フラグメントが存在しないため、免疫沈降サンプルの酵素消化、還元、溶出を実施する際に、試薬由来の重鎖や軽鎖フラグメントがコンタミネーションすることがありません(使用製品:GFP-Trap® Agarose)。VHH抗体(Nanobody®)は高い結合親和性を示し、コンパクトかつ頑健な構造を有するため、プルダウン時や精製時に厳しい洗浄条件を適用できます(クロモテック製品の結合親和性:1 pM~100 nM)。
クロモテックのGFP-Trap® Agarose、GFP-Trap® Magnetic Agarose、GFP-Trap® Magnetic Particles M-270のような、ビーズにVHH抗体(Nanobody®)が結合したReady-to-useな製品群は、様々なターゲットに利用可能です。Nano-Trapシリーズの製品に関する詳しい情報はこちらをご覧ください。
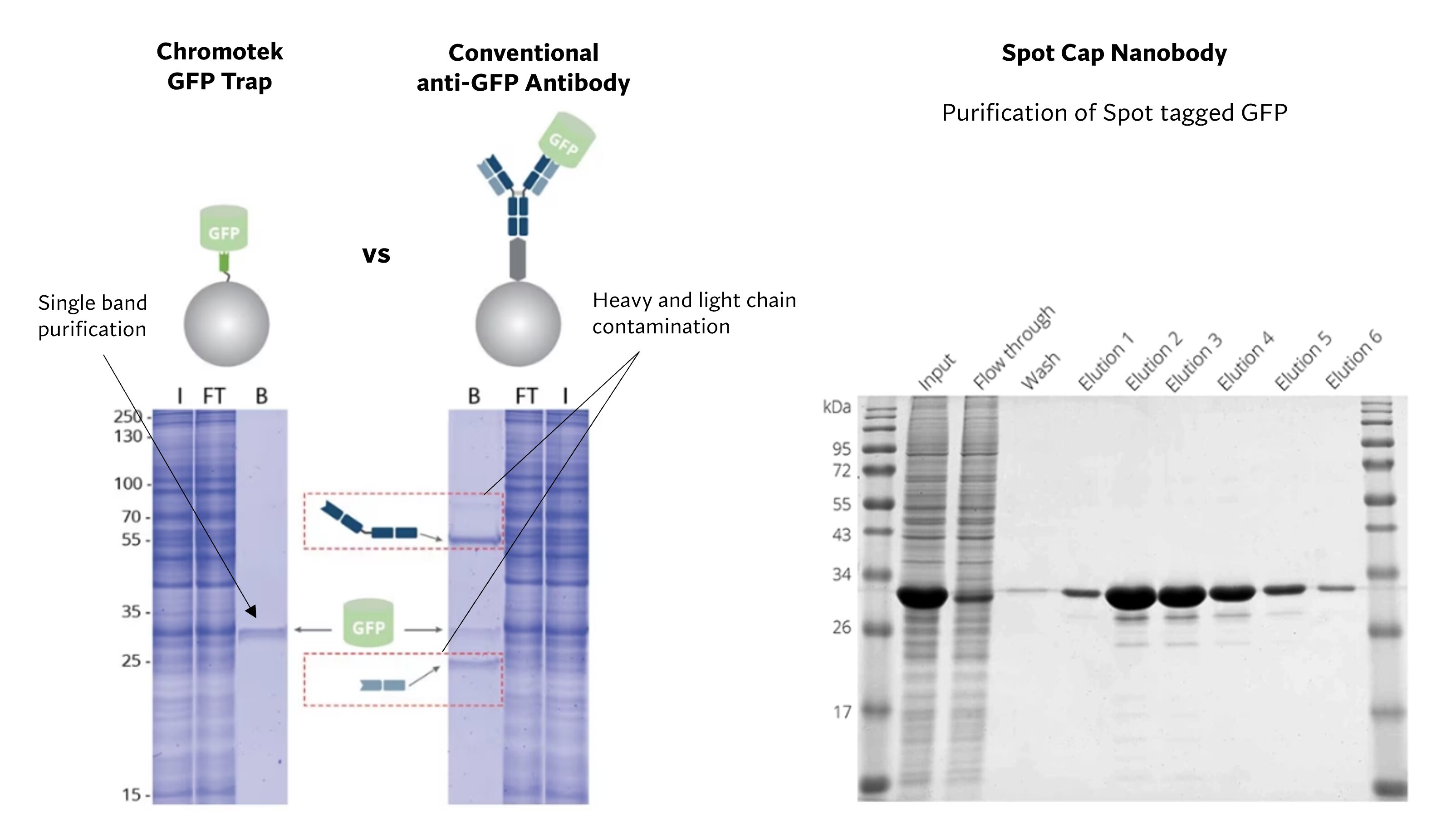
図4. プロテインテックのVHH抗体(Nanobody®)を使用したGFPタンパク質のプルダウンと精製。左)クロモテックのGFP-Trap®と従来型抗体結合ビーズの比較。GFP-Trap®を使用したプルダウンサンプルは、単一のバンドが検出されます。一方、従来型抗体結合ビーズを使用したサンプルは、IgG抗体の重鎖・軽鎖フラグメント由来のコンタミネーションも検出されます。右)Spot-Cap®を使用したSpot-Tag®融合GFPタンパク質の精製。Nano-Trapシリーズの製品は特異性が高く高親和性であるため、1回の精製で純度の高いGFPタンパク質を得られます。
同一種由来の抗体を使用したマルチプレックス染色
プロテインテックのVHH抗体(Nanobody®)はエピトープに対する特異性が高く、クロモテックのNano-Secondary®試薬は異なるIgGサブタイプを認識します。そのため、同一種由来の一次抗体を使用してマルチプレックス染色を実施できます(適用可能なアプリケーション:IF・IHC・SRM・FC・WB)。図5には、3種類のマウスIgGサブクラス(IgG1・IgG2b・IgG3)特異的なクロモテックのNano-Secondary®を使用したHeLa細胞のマルチプレックス染色画像を示しました。Nano-Secondary®は特異性が高いため交差反応を低く抑えることができます。その結果、従来型二次抗体と比較して、バックグラウンドが低くS/N比(シグナル/ノイズ比)の良好なシグナルを得ることができます。
プロテインテックの関連製品:Nano-Secondary®
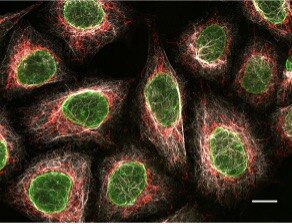
図5. 3種類の異なるサブクラス特異的なNano-Secondary®を使用したHeLa細胞のマルチプレックス免疫蛍光染色。灰色:抗ビメンチン マウスIgG1抗体+Alexa Fluor® 647標識抗マウスIgG1 VHH抗体。緑:抗ラミン マウスIgG2b抗体+Alexa Fluor® 488標識抗マウスIgG2b VHH抗体。赤:抗MOTマウスIgG3抗体+Alexa Fluor® 568標識抗マウスIgG3 VHH抗体。撮影:ルートヴィヒ・マクシミリアン大学ミュンヘン(LMU-Munich)Core Facility Bioimaging部門(スケールバー:10 μm)。
Nanobodyの制約事項
何らかの利点があるということは、その他の利点を享受できないということでもあります——プロテインテックのVHH抗体(Nanobody®)には様々な利点がありますが、いくつかの欠点も存在します。
明るさ/蛍光色素の標識度(DOL)
通常のプロテインテックのNano-Secondary®製品には、Nano-Secondary®1分子につき2分子の蛍光色素が共有結合しています。Nano-Secondary®がターゲットとするFcフラグメント鎖1本につき、2~4分子のVHH抗体(Nanobody®)が結合するため、1分子の一次抗体にはおよそ4~8分子の蛍光色素が付加することになります。必然的に、VHH抗体(Nanobody®)を一次抗体として使用する場合は、シグナル強度に関して分子量と明るさに関係する制限が生じます(該当製品:Nano-Booster & Nano-Label)。
従来のアプローチでは、ターゲットとなる特定の動物種由来の一次抗体に対してポリクローナル二次抗体を使用します。従来型抗体はVHH抗体(Nanobody®)よりも分子量が10倍ほど大きく、二次抗体1分子あたりのDOLの値も大きくなりシグナルも明るくなります。この効果は一次抗体の複数のエピトープに結合するというポリクローナル二次抗体の特性(Polyclonality)によってさらに増幅されます。
では、何故一次抗体としてVHH抗体(Nanobody®)が販売されているのでしょうか?それは、すべてのアッセイが明るさを第一条件とするわけではないためです。例えば、SRMのように解像度の高さが求められる場合が挙げられます。SRMを実施する場合は、エピトープ‐抗体間の距離が短い方がより正確なイメージング画像を得られるため、VHH抗体(Nanobody®)の方がより良い選択肢となります。分子量が小さいというプロテインテックのVHH抗体(Nanobody®)の利点は、特に核のような細胞内の混雑環境下で発揮され、良好な組織透過性を示し、細胞をより均一に染色することができます。
強度の強い蛍光シグナルを必要とする場合や、シグナル増幅する必要があるその他のアッセイの場合は、従来型IgG抗体を選択した方が良いでしょう。どのような抗体を使用するかは実験の内容と目的によって異なります。VHH抗体(Nanobody®)や従来型抗体の特性や制約が実験にどのように影響を及ぼすか十分に考慮しましょう。
Note:現在、プロテインテックはVHH抗体(Nanobody®)製品のDOLや明るさを改善するべく、様々なソリューションの開発に取り組んでいます。
最後に
本稿をご覧いただいた皆様には、プロテインテックのVHH抗体(Nanobody®)試薬は複雑な試薬ではなく、前衛的でニッチな製品ではないことがおわかりいただけるのではないでしょうか。VHH抗体(Nanobody®)試薬は、数多くの利点を提供し、入手が容易で使用しやすく、実践的で汎用性が高く、極めて優れたツールであることから、あらゆるラボに備えておく価値のある製品といえるでしょう。
VHH抗体(Nanobody®)試薬は、従来型IgG抗体と比較して分子量が小さく、良好な組織/細胞透過性を示し、高解像度の撮影を実現するといった、従来型の一次抗体にはない利点を提供するため、特にイメージングアプリケーションを実施するうえで有益な試薬です。さらに、高い特異性と強い親和性を示し、重鎖と軽鎖の存在しない構造を有することから、様々な免疫沈降アッセイに最適な試薬です。
端的に述べれば、プロテインテックのVHH抗体(Nanobody®)は従来型抗体の代替品以上の価値があり、この分野の著しい進歩を象徴する製品です。VHH抗体(Nanobody®)の示す数々の利点は、研究の効率性と成果を大いに高めることができるでしょう。VHH抗体(Nanobody®)は、携わる研究分野を問わず、ラボワークにそのまま取り入れることのできる最新のソリューションを提供します。
参考文献
S Muyldermans. A guide to: generation and design of nanobodies. FEBS J. 2021 Apr;288(7):2084-2102.
ChromoTek GFP-Trap® Agarose(ptglab.co.jp)
Philipp Becker博士著(プロテインテック/クロモテック Product Manager)2024年7月
関連情報
GFP-Trap for immunoprecipitation
Why GFP-Trap gives the best immunoprecipitation results
What GFP-Trap should I use for my immunoprecipitation?
On-bead digestion protocol: From immunoprecipitation with nanobodies to MS analysis
VHH抗体(別名:Nanobody®)に蛍光色素を標識する方法
Understanding Liver Cancer: Causes, diagnostics, and therapeutic avenues


