IF Signal-To-Noise-Ratios
Autofluorescence
Autofluorescence complicates the analysis of IF stainings. In general, the spectra of autofluorescence are very broad, compared to those of fluorescent probes. Thus, it is challenging to distinguish between autofluorescence and the actual fluorescence of interest, leading to misunderstanding of the image analysis. To overcome the problem of autofluorescence, it is important to have an idea of its source before starting to optimize the protocol or playing around with the filter sets. Autofluorescence is often used as a collective term for “biological autofluorescence” or so-called “fixativeinduced autofluorescence.”
Biological Autofluorescence
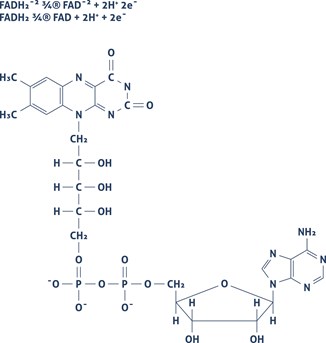
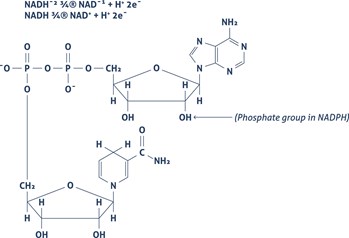
Cells contain components that show fluorescent when excited by a suitable wavelength. This biological autofluorescence has its origin in endogenous fluorophores. Biological autofluorescence mainly comes from mitochondria, lysosomes, and aromatic amino acid components. The most important components causing intrinsic fluorescence are Flavin coenzymes (FAD, FMN) (Figure 1) or pyridine nucleotides (NADH) (Figure 2).
| Figure 1 | Figure 2 |
 |
 |
Fixative-induced Autofluorescence
When fixing with aldehydes, these aldehydes can react with cellular amines or proteins, resulting in a fluorescent product. These issues can be diminished by reducing the aldehyde group to a hydroxyl group (e.g., by sodium borohydride).
Most Commonly Used Fixative For IF Staining

