Selecting Antibodies For IHC
Selecting And Optimizing Antibodies For IHC
When deciding on an antibody, the following points should be considered to increase the likelihood of high specificity and low cross-reaction and a successful staining; further optimization will then help to achieve a reliable signal: Consulting literature and antibody comparison resources, checking in-house validation data of the antibody seller, searching for independent validation data, and consulting the original manufacturer for technical service and protocol support.
Selecting a primary antibody for IHC
The initial choice of the primary antibody can affect the whole outcome of the experiment. Thus, it is important to search for an antibody that is well validated to increase the chance of a successful experiment. Most importantly, it is helpful to purchase the antibody from the original manufacturer and not from a vendor, as only the original manufacturer has access to all validation data and can assist with superior technical support.
Polyclonal Vs. Monoclonal Antibodies
Benefits of polyclonal antibodies in IHC
- Heterogenous population.
- Recognise multiple epitopes.
- Stable to changes (pH, tissue, buffer, protein confirmation), stable detection.
Drawbacks of polyclonal antibodies in IHC
- Not specific to one epitope.
Benefits of monoclonal antibodies in IHC
- Homogenous population.
- Specific to a single epitope.
- Detects a single protein with high affinity, even though it shares sequences/similarities with other proteins.
Drawbacks of monoclonal antibodies in IHC
- Sensitive to even small changes (pH, tissue, buffer, protein confirmation), no stable detection.
Optimizing A Primary Antibody For IHC
General steps on optimizing conditions for a primary antibody in IHC
- Keep incubation time and temperature, titrate different antibody dilutions.
- Specific staining, but background signal: Vary incubation time and temperature for a short time.
- High-affinity antibody with a high concentration: Incubation with a high concentration for a short time.
- High-affinity antibody with a low concentration: Increase incubation time, lower incubation temperature.
- Polyclonal antibodies, in general, can be used at a higher working dilution than monoclonal antibodies.
Selecting And Optimizing Secondary Antibodies For IHC
Subclass specificity
Polyclonal primary antibodies are mainly IgG isotypes. Primary monoclonal antibodies are occasionally of a different isotype and therefore need an isotype-specific antibody.
Cross-absorption
Secondary antibodies can go through an additional purification step to reduce potential cross-reactions with other species. Therefore, the secondary antibody solution is passed over different columns containing sera proteins of different species to filter out the non-specific secondary antibody.
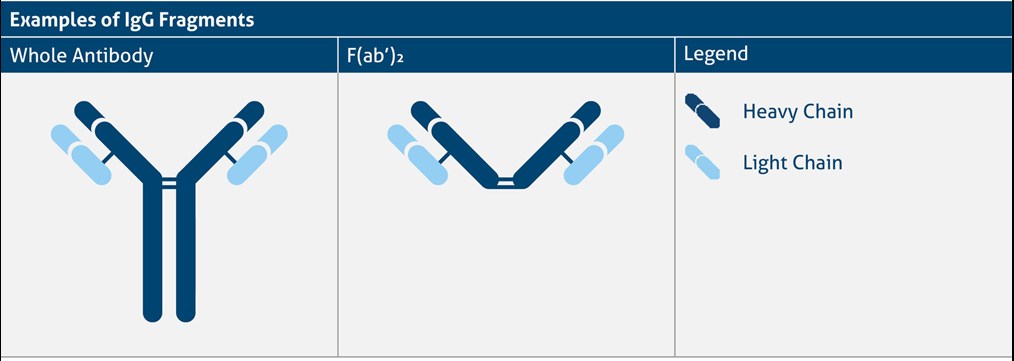
F(ab’)₂ fragments
High background staining can be due to the presence of Fc receptors in special tissue or cells.
IHC Controls
Positive controls
- Control tissue that is known to express the protein of interest will prove that the staining protocol and antibody are working properly.
Negative controls
- Control tissue that is known not to express the protein of interest will ensure that the observed staining pattern is due to specific signals.
- Incubate the tissue with the secondary antibody only. This control ensures that no cross-reactions or non-specific background signals are observed due to the secondary antibody and the detection reagents.
- Observing the unstained tissue under the microscope in the brightfield/fluorescence channel gives an idea about biological background signal/autofluorescence that mainly comes from mitochondria, lysosomes, and aromatic amino acid components and could be misinterpreted with positive staining.
- Incubating the tissue with a non-immune immunoglobulin of the same isotype will ensure that the observed staining is not due to unspecific binding of immunoglobulins.

