Scientists Discover Dynein-Driven Motility in Primary Cilia!
Researchers have discovered that the movement of primary cilia on pancreatic cells assists in insulin secretion
Cilia Structure and Function
Cilia are hairlike membrane-bound organelles that are found on the surface of nearly all mammalian cells. Broadly, cilia can be categorized into two groups: primary cilia and motile cilia.
Primary Cilia: Most cells contain one primary cilium (thought to be stationary) that acts as an antenna to sense the extracellular environment and help mediate cell signaling cues. The structure of most primary cilia consists of an axoneme containing nine microtubule pairs (9+0 axoneme, see Fig. 1). Embryonic defects in primary cilia can lead to developmental issues, such as abnormalities of the heart, bone, and brain (PMID: 24296655). Primary cilia dysfunction has also been associated with cancer, obesity, diabetes, cyst formation, and cognitive disorders (such as schizophrenia, bipolar disorder, and Alzheimer’s disease) (PMID: 29030052).
Motile Cilia: In comparison, motile cilia are present in large numbers on select cell types and beat in coordinated waves. For example, motile cilia found in the respiratory tract function to clear mucus containing debris away from the lungs. Like primary cilia, the structure of motile cilia generally consists of an axoneme containing nine microtubule pairs. However, motile cilia also usually contain a central pair of microtubules (9+2 axoneme). Furthermore, the motile cilia structure includes outer and inner dynein arms and radial spokes (see Fig. 1) to aid in movement. Dysfunction in motile cilia structure and function can lead to primary ciliary dyskinesia and can result in sinusitis, bronchiectasis, and infertility (PMID: 16036877).
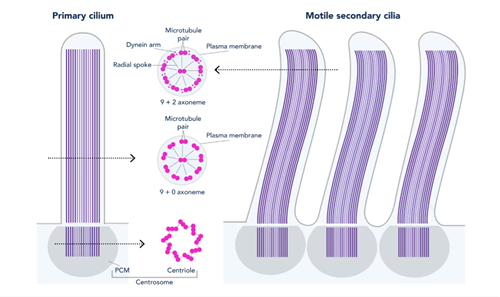
Figure 1. Structure of primary and motile secondary cilia
A New Discovery: Motile Primary Cilia in Pancreatic Islets
Structure of Motile Primary Cilia: Primary cilia have historically been considered stationary. However, researchers at Washington University in St. Louis recently made a serendipitous discovery while studying cilia on pancreatic islets. The study, published in Science by Cho and colleagues (PMID: 36149960), used transmission electron microscopy to identify that human islets contain primary cilia that diverged from the traditional 9+0 microtubule arrangement. Instead, they observed an axoneme containing only eight microtubule pairs with an additional central doublet or singlet. Furthermore, the scientists identified structures resembling inner and outer dynein arms and radial spokes, suggesting that these primary cilia had the machinery necessary for motility. These findings were confirmed by immunofluorescence identifying key motile cilia proteins. Proteintech’s ARL13B (17711-1-AP) and Acetylated Tubulin (AcTUB, 66200-1-Ig) antibodies were used for immunostaining of cilia in this study!
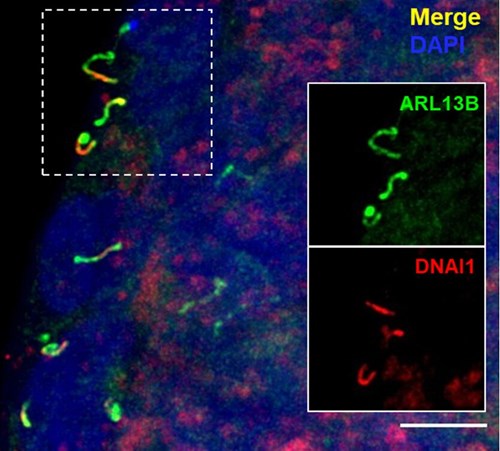
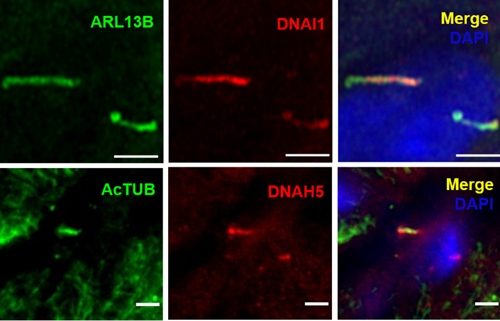
Figure 2. Selected images from Cho et al. (CC BY 4.0), identifying characteristics of motile primary cilia in human islets. Dynein (DNA1) motor proteins are expressed in ARL13B-positive primary cilia (top). High magnification images of DNA1 motor proteins expressed in ARL13B-positive primary cilia (bottom, top row) and DNAH5 dynein arm component expressed in acetylated tubulin (AcTUB)-positive primary cilia (bottom, bottom row).
Cilia Movement Depends on Glucose, Dynein, and ATP: After identifying that islet primary cilia express motor proteins, the researchers analyzed whether this translated to cilia motility. They generated a mouse model containing fluorescent cilia in pancreatic beta cells and identified spontaneous movement of islet beta cell cilia using live cell imaging. These primary cilia moved differently from conventional motile cilia with slower waves and more circular movements. Using waveform tracing to quantify cilia movements, the researchers observed that when environmental glucose was increased, cilia movement intensified.
Additionally, genetic knockdown studies and pharmacologic inhibition of axonemal dynein in mouse islets resulted in cilia immobility, suggesting that cilia movement is dynein-dependent. Interestingly, when islets were cultured with exogenous ATP, without glucose stimulation, there was a significant increase in cilia movement that diminished in the presence of ATP inhibitors. These findings suggest that beta cell cilia move using a dynein-driven system dependent on ATP for energy.
Cilia Movement Regulates Insulin Release: The researchers next sought to characterize the biological function of cilia movement. In pancreatic beta cells, calcium dynamics is an important player in glucose-regulated insulin release. The findings from this study showed that knockdown of axonemal dynein in islet beta cells reduced cytosolic Ca2+ in response to glucose, resulting in decreased insulin secretion measured by a static Glucose-stimulated Insulin Secretion assay. These findings were observed in both human and mouse islets.
What this means for the future: Cilia of islet cells play an important role in glucose-dependent hormone release and, as a result, are associated with metabolic disorders such as obesity and diabetes. These groundbreaking findings from Cho et al., suggest that alterations in cilia motility might result in altered insulin secretion and could potentially act as a new therapeutic target to treat metabolic diseases.
Find out more about the Proteintech antibodies that were used to make this groundbreaking discovery!
ARL13B (17711-1-AP)
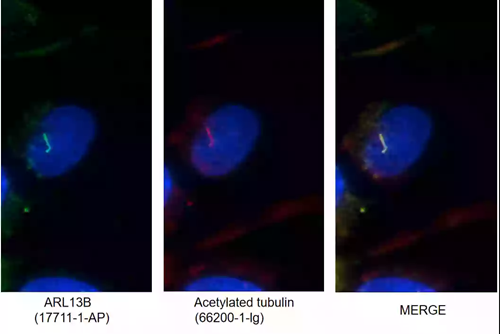
Figure 3.I F images of MDCK cells stained with ARL13B rabbit pAb (17711-1-AP, left) and acetylated tubulin mouse mAb (66200-1-Ig, middle). Merged image (right) shows co-localization in primary cilia.
ARL13B Background Information: ARL13B, also called ARL2L1, is a small ciliary G protein of the Ras superfamily. Localized in the cilia, it is required for cilium biogenesis and sonic hedgehog signaling. Defects in ARL13B are the cause of Joubert syndrome (JS), which is an autosomal recessive disorder characterized by a distinctive cerebellar malformation (PMID: 19906870).
Acetylated Tubulin (AcTub, 66200-1-lg)
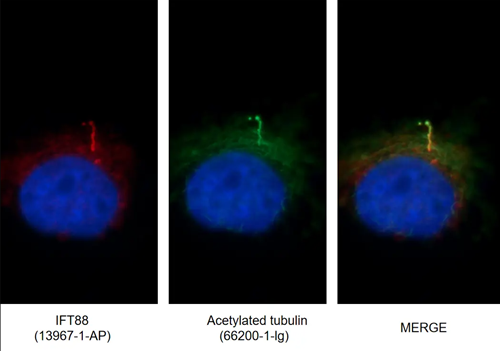
Figure 4. IF images of MDCK cells stained for IFT88 rabbit pAb (13967-1-AP, left) and acetylated tubulin mouse mAb (AcTub, 66200-1-Ig, middle). Merged image (right) shows co-localization in primary cilia.
Acetylated Tubulin Background Information: Acetylation is a conserved post-translational modification of alpha tubulin at Lys 40 during tubulin assembly, and it correlates to increased microtubule stability and intracellular transport (PMIDs: 29207274, 30079247, 20940043). Acetylated-α-tubulin is located in cytoplasmic tubulin as well as in cilia; therefore, it is not strictly region-specific (PMID: 30079247).
Interested in other cilia targets? Browse all our cilia antibodies here.
Related Content
Imaging the Rainbow: Multiplexing with Same-Species Antibodies for Immunofluorescence
Primary Cilia and Regulation of Blood Pressure
Second Cancer After Breast Cancer

Support
Newsletter Signup
Stay up-to-date with our latest news and events. New to Proteintech? Get 10% off your first order when you sign up.


