Validation Data Gallery
Product Information
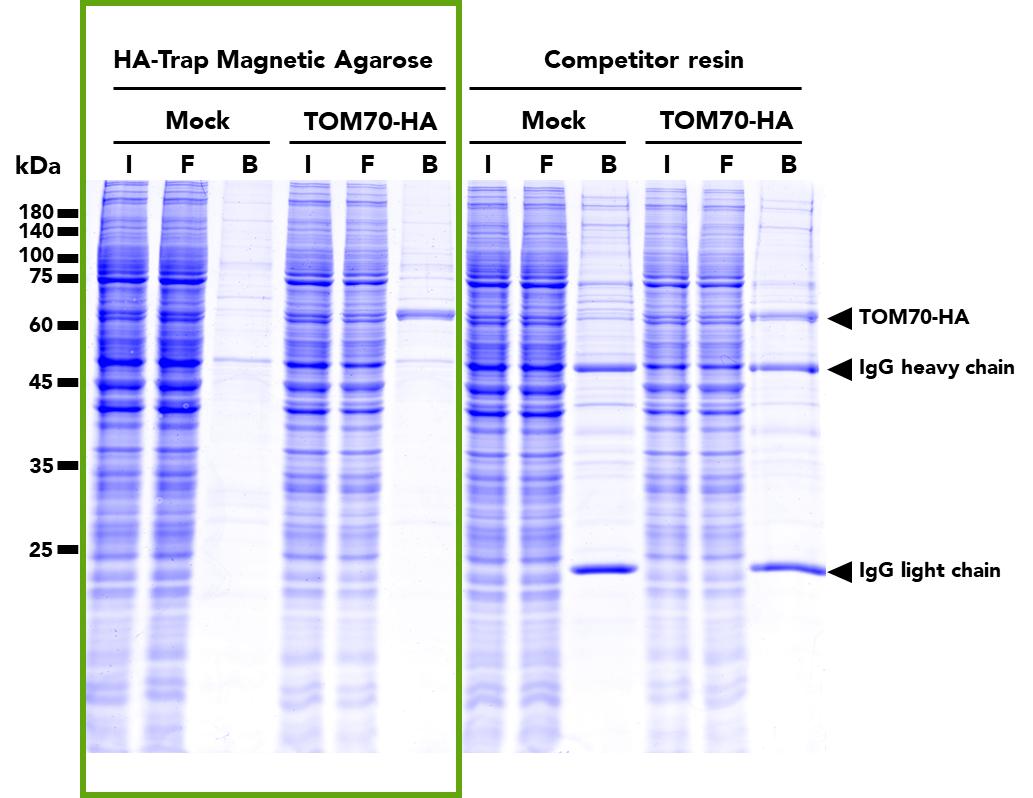
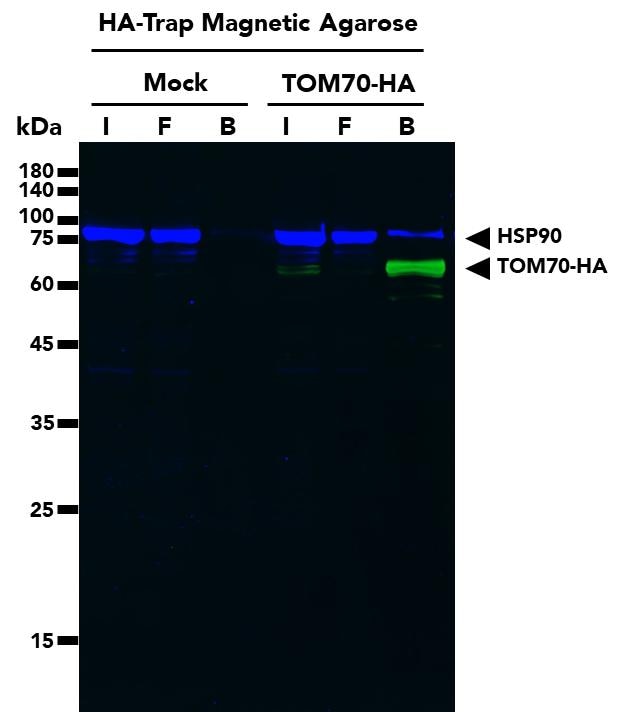
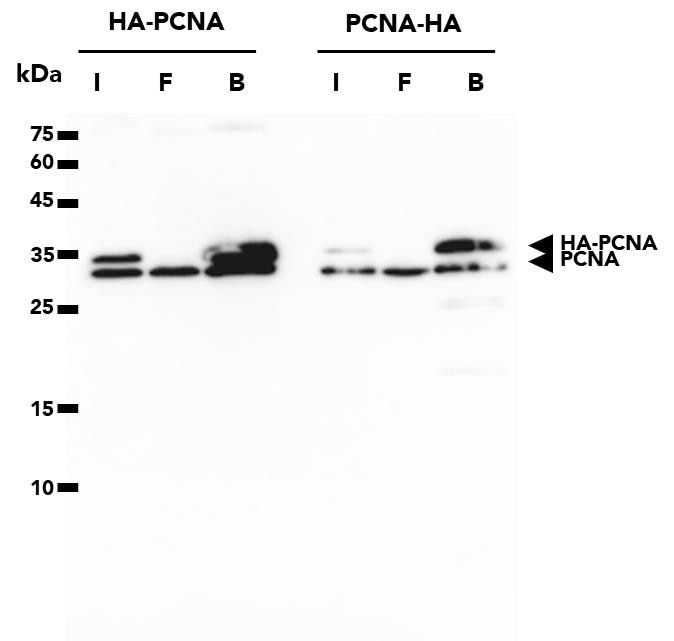
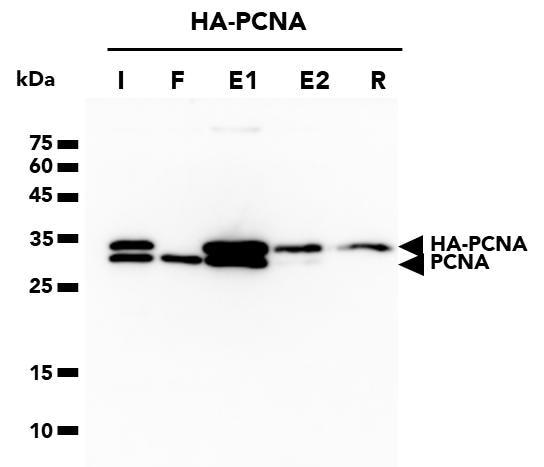
The ChromoTek HA-Trap Magnetic Agarose consists of an anti-HA-tag Nanobody/VHH, which is coupled to magnetic agarose beads. It can be used for the immunoprecipitation of HA-fusion proteins from cell extracts of various organisms such as humans, mice, dogs, plants, and yeast.
| Description | Immunoprecipitation of HA-fusion proteins with anti-HA Nanobody conjugated to magnetic agarose beads. • Fast, reliable & efficient one-step immunoprecipitation • Ready-to-use • No heavy & light antibody chains • Stable under harsh washing conditions • Suitable for downstream mass spec analysis • Works in samples from: mammals, plants, yeast, etc. |
| Applications | IP, Co-IP |
| Specificity/Target | Binds specifically to the HA-tag (sequence YPYDVPDYA) fused to a protein of interest at N-, C- or internal position. Please note that the affinity is highest for a C-terminal fusion. There is no cross-reactivity to other common peptide tags such as the His6-tag, FLAG-tag, Spot-Tag, V5-tag, Strep-tag, or C-tag (other tags not tested). Background binding to host cell proteins from a range of organisms such as human, mouse and dog cell lines or yeast and plants is low. |
| Binding Capacity | 20 ug recombinant HA-tagged protein (~30 kDa) per 25 uL bead slurry |
| Conjugate | Magnetic agarose beads; ~40 um (cross-linked 6% magnetic agarose beads) |
| Elution buffer | 2x SDS-sample buffer (Lammli) |
| Wash Buffer Compatibility | 2M NaCl, 5 mM DTT, 0 mM β-mercaptoethanol, 5 mM TCEP, 2% NP40, 2% Triton X-100, 0% SDS, 2-3 M Urea |
| Type | Nanobody |
| Class | Recombinant |
| Host | Alpaca |
| Affinity (KD) | 6 nM for C-terminal HA-tags and ca. 180 nM for N-terminal fusions. |
| Compatibility with mass spectrometry | The HA-Trap is optimized for on-bead digestion. For the application note, please click here: On-bead digest protocol for mass spectrometry |
| RRID | AB_3094561 |
| Storage Buffer | 20% ethanol |
| Storage Condition | Shipped at ambient temperature. Upon receipt store at +4°C. Stable for one year. DO not freeze! |
| Size | 25ul/reactions (eg:20rxns=500ul slurry) |
Publications
| Application | Title |
|---|---|
Mol Plant The OsSRO1c-OsDREB2B complex undergoes protein phase transition to enhance cold tolerance in rice | |
Plant Cell Maize COMPACT PLANT 3 regulates plant architecture and facilitates high-density planting | |
Plant Physiol ABSCISIC ACID-INSENSITIVE 5-KIP-RELATED PROTEIN 1-SHOOT MERISTEMLESS modulates reproductive development of Arabidopsis |