マクロファージ分極化入門:基礎と実験手順
Consuelo Coser著(Nottingham大学、博士課程在籍)
マクロファージの分極化(極性化)とは、特定の外的刺激によって単球が機能的に異なるマクロファージ表現型(M1、M2)へ分化誘導される免疫応答の過程を指します。分極化したマクロファージは、生体を脅かす様々な脅威に対抗します。
|
目次 |
免疫応答
免疫系の目的は、生体の健康を危機に陥れる可能性のある病原体や有害物質から身体を保護することにあります。免疫系には自然免疫応答と獲得免疫応答が関与し、両者が協調することで、病原体を排除し、毒素やアレルゲンを無毒化し、有害となり得るあらゆる因子に対処します。
自然免疫系(Innate immune system)
自然免疫系は、病原体に対して最初に機能する生体防御機構です。自然免疫系には、皮膚、体温、生理活性分子、病原体表面等に存在する特異的な分子パターンを標的とする細胞膜受容体/細胞質タンパク質等の物理的・生理的バリアが挙げられます。病原体に遭遇すると自然免疫系は速やかに活性化します。また、その防御機構は非特異的で、免疫記憶を示さないという特徴があります。
獲得免疫系(Adaptive immune system)
自然免疫系とは異なり、獲得免疫応答は、特定の抗原の認識、自己/非自己抗原の識別、抗原特異的応答の展開という過程が関与するため、病原体の侵入から免疫応答成立までの時間差があることを特徴とします。さらに、獲得免疫系には免疫記憶という機構があり、一度暴露されてから再度同一の病原体に暴露された際に、より迅速かつ効率的に免疫応答を開始します1,2。
マクロファージの役割
マクロファージは自然免疫系のエフェクター細胞であり、免疫応答において大きな役割を果たします。マクロファージは、貪食作用と分解作用によって、細胞デブリ、死細胞、損傷組織等の除去を行うスカベンジャー細胞で、微生物、移植片・移植物のような「非自己」と認識した物質に対して貪食を試みます。そのため、移植手術を実施する際は生体内移植器具の大きさや素材等を、貪食作用等の異物反応による線維性被膜化といった悪影響が生じないように設計する必要があります3。マクロファージは異なる表現型に分極化する能力があり、炎症誘発反応と抗炎症反応のいずれの応答にも関与します。マクロファージは、炎症性サイトカイン・抗炎症性サイトカインに加え、活性酸素種(ROS:Reactive oxygen species)、一酸化窒素(NO)4、場合によってはミエロペルオキシダーゼ(MPO:Myeloperoxidase)等の宿主防御を担う抗微生物性メディエーターも分泌します。
マクロファージの分極化機構
マクロファージは血液を循環する単球に由来し、必要とされる組織へ単球が遊走してその場の状況に応じてマクロファージに分化します。生体内の条件や受ける刺激に応じて、単球は異なる経路を辿り、M0、M1、M2型として知られる異なる分極化状態のマクロファージに分化します。マクロファージの表現型に影響を及ぼす様々なサイトカインや増殖因子のなかでも、マクロファージコロニー刺激因子(M-CSF:Macrophage colony-stimulating factor)は単球の増殖・分化・機能の活性化を制御する中枢的なサイトカインです。
プロテインテックの関連製品:HumanKine®(ヒューマンカイン)組換えM-CSF
M0型のマクロファージは未分極のマクロファージ(Naïve macrophage)で、炎症誘発性刺激にも抗炎症性刺激にも暴露されていない状態のマクロファージを指します。
典型的な活性化を受けたマクロファージはM1型マクロファージと呼ばれ、リポ多糖(LPS:Lipopolysaccharide)、IFN-γ、顆粒球マクロファージコロニー刺激因子(GM-CSF:Granulocyte-macrophage colony-stimulating factor)、IL-2、IL-12、IL-18、TNF-α、TNF-β等によって誘導・活性化されます。M1型マクロファージは炎症誘発性細胞であり、炎症性サイトカイン、NO中間体やROS中間体5、リゾチームや中性プロテアーゼ等の酵素を産生・放出するほか、微生物等の病原体を分解します6。
プロテインテックの関連製品:HumanKine®組換えIFN gamma
HumanKine®組換えGM-CSF
HumanKine®組換えIL-2
HumanKine®組換えIL-12
HumanKine®組換えTNF-α
IL-18関連製品
TNF-β関連製品
M1型活性化の経路とは別の活性化経路を辿ることで、マクロファージはM2型マクロファージに分化します。M2型に分化するとマクロファージは、IL-10、マトリックスメタロプロテアーゼや、血管内皮増殖因子(VEGF:Vascular endothelial growth factor)7、TGF-β1、PDGF等の増殖因子を放出します8。さらに、M2型マクロファージはより高い貪食能を特徴とします。M2型マクロファージは、IL-4、IL-6、IL-10、IL-13等のサイトカインの他に、糖質コルチコイド等による刺激で誘導・活性化されます。
プロテインテックの関連製品:HumanKine®組換えIL-4
HumanKine®組換えIL-6
HumanKine®組換えIL-10
IL-13関連製品
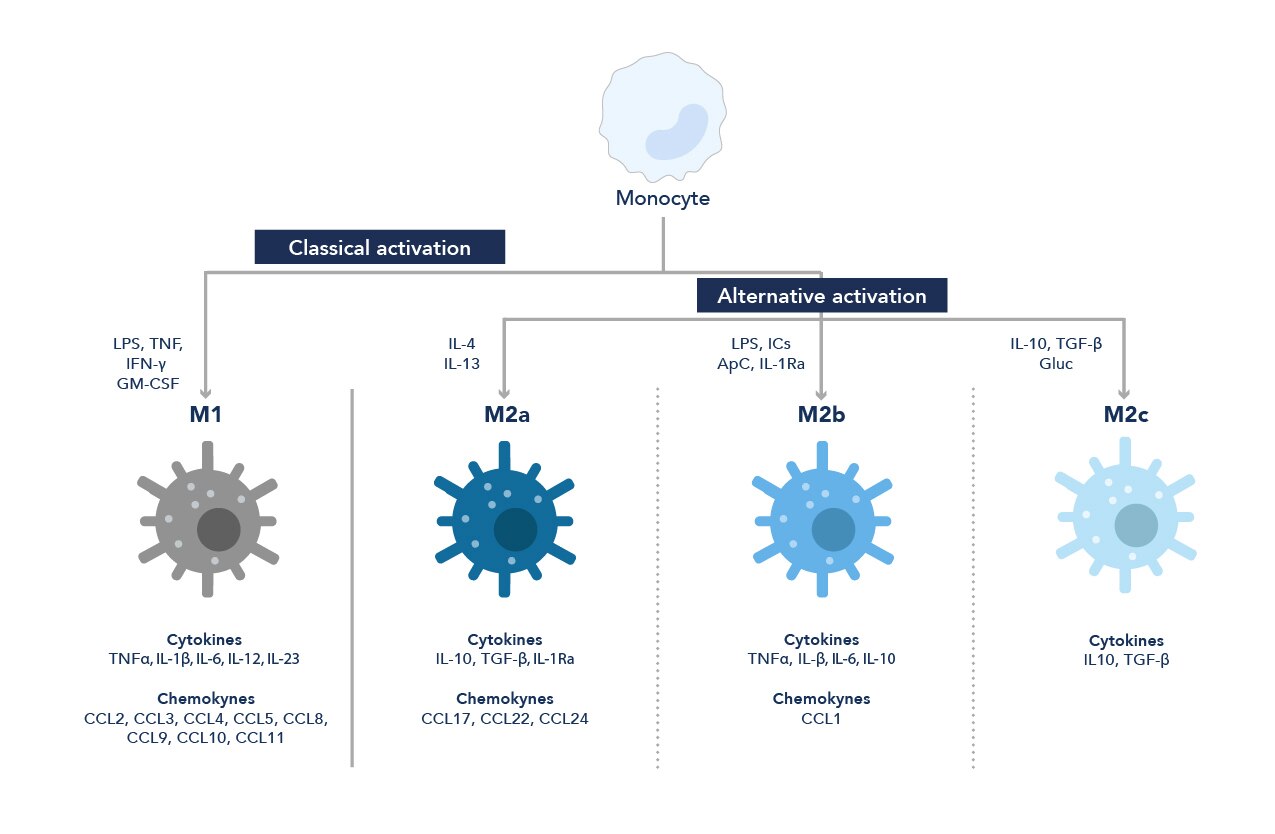
図1. 単球からマクロファージへの分化経路
マクロファージは受けた刺激に応じて、炎症誘発作用(M1)、または抗炎症作用(M2)のいずれかの作用を示します。
通例、マクロファージはM1型とM2型の2種類の代表的な系統に分類されるものの、周辺の微小環境に応じて様々なマーカーを発現し、サブタイプごとに特徴的な表現型を示します。マクロファージは極めて可塑性が高く挙動が大きく変化する細胞で、マクロファージ表現型は様々な刺激に応答して経時的に変化します9。
マクロファージ研究:実験ワークフロー
異物反応等の免疫応答や生体応答におけるその役割の重要性から、マクロファージ研究には大きな意義があります。以下のセクションでは、単球の単離と分化のワークフローとともに、マクロファージの特性解析について紹介します。
単球の単離
単球は、ポジティブセレクションまたはネガティブセレクションを実施することで、全血サンプルから単離できます。ポジティブセレクションでは、目的の種類の細胞を選別するために特異的な細胞表面抗原を標的とします。例えば、CD14は単球上に発現する細胞表面抗原で、単球を単離する場合はヒトCD14と特異的に結合する磁気ビーズ等を使用します10。CD14によるポジティブセレクションを実施する場合、プロテインテックの磁気ビーズ「Human CD14 Magnetic Beads(カタログ番号:MS006)」等を利用することができます。一方、ネガティブセレクションでは、単球だけが残るまで不要な細胞群を除去します。この分離手法を採用する場合、目的の細胞を分離できる細胞分離用磁気ビーズキットを用意する必要があります。
プロテインテックの関連製品:CD14関連製品
細胞分離用磁気ビーズキット
単球からM0、M1、M2型マクロファージへの分化プロトコール10
注意:すべてのステップは、滅菌処理等の手技を用いて無菌環境下で実施してください。本稿では、24ウェルプレートを使用した単球分化実験を想定したプロトコールを紹介します。
用意するもの:FBS(10% v/v)、L-グルタミン(1% v/v)、ペニシリン‐ストレプトマイシン(1% v/v)を添加したRPMI-1640培地。M-CSF、GM-CSF、IFN-γ、IL-4
プロトコール
Day 1
1. 健常ドナー由来の血液から単球を分離し、3.5~6 x10^6個の単球を培地(12 mL)に再懸濁します。
2. 3種類の分化マクロファージを作製するために、単球懸濁液を3本のファルコンチューブに分注します(各4 mL)。
3. 作製したい表現型に応じて、各ファルコンチューブに以下のサイトカイン(表1)を添加します。
表1. 添加するサイトカインとその濃度
単球を異なる表現型のマクロファージに分化誘導するために、RPMI-1640培地に目的の表現型に応じたサイトカインを添加します。
|
表現型 |
添加サイトカイン |
最終濃度(ng/mL) |
|
M0 |
10 |
|
|
M1 |
20 |
|
|
20 |
||
|
M2 |
50 |
|
|
20 |
注意:添加するサイトカイン溶液の液量は、使用するストック溶液の濃度によって異なります。
必要な液量はこちらの計算式を使用して計算してください:Ci x Vi = Cf x Vf
Ci=ストック溶液濃度、Cf=最終濃度、Vi=ストック溶液液量、Vf=最終液量
4. サイトカイン添加後、おだやかに混和し24ウェルプレートに播種します(1 mL/ウェル)。
5. 37℃、5% CO2条件下でインキュベーションします(~Day 3)。
Day 3
1. 培地を交換します。Day 1で使用したサイトカインと培地を同量使用します。
2. 37℃、5% CO2条件下でインキュベーションします(~Day 7)。
マクロファージの特性評価
Day 7に分化プロセスは完了します。マクロファージを4% PFA(パラホルムアルデヒド)で15分間固定し、様々な解析手法で特性評価を実施します。
a. 形態解析
図2に示す通り、マクロファージの形態は表現型によって変化します。M1型マクロファージやM2型マクロファージとは異なり、ナイーブマクロファージ(M0型)は丸い形状を特徴とします。この培養条件では、M1型マクロファージは細長い形状を示し、M2型マクロファージは丸い形状でクラスターを形成しています。

図2. 単球(Day 1、Day 3)とマクロファージ(Day 6)の画像。単球を組織培養プラスチックプレート上で培養し、M0型、M1型、M2型マクロファージに分化させたサンプルを撮影しました(スケールバー:20 µm)。
b. 免疫蛍光染色(IF:Immunofluorescence)
M1型マクロファージとM2型マクロファージは、カルプロテクチンとマンノース受容体(MR:Mannose receptor)の発現パターンに違いが認められます。
カルプロテクチンはM1型マクロファージ、マンノース受容体はM2型マクロファージのマーカーです。異なる蛍光色素を標識したカルプロテクチン抗体とMR抗体を使用すると、免疫蛍光イメージングによってマクロファージ表現型を解析することができます。
蛍光染色による定量を実施する場合は、撮影画像内の細胞数が一定になるよう留意します。
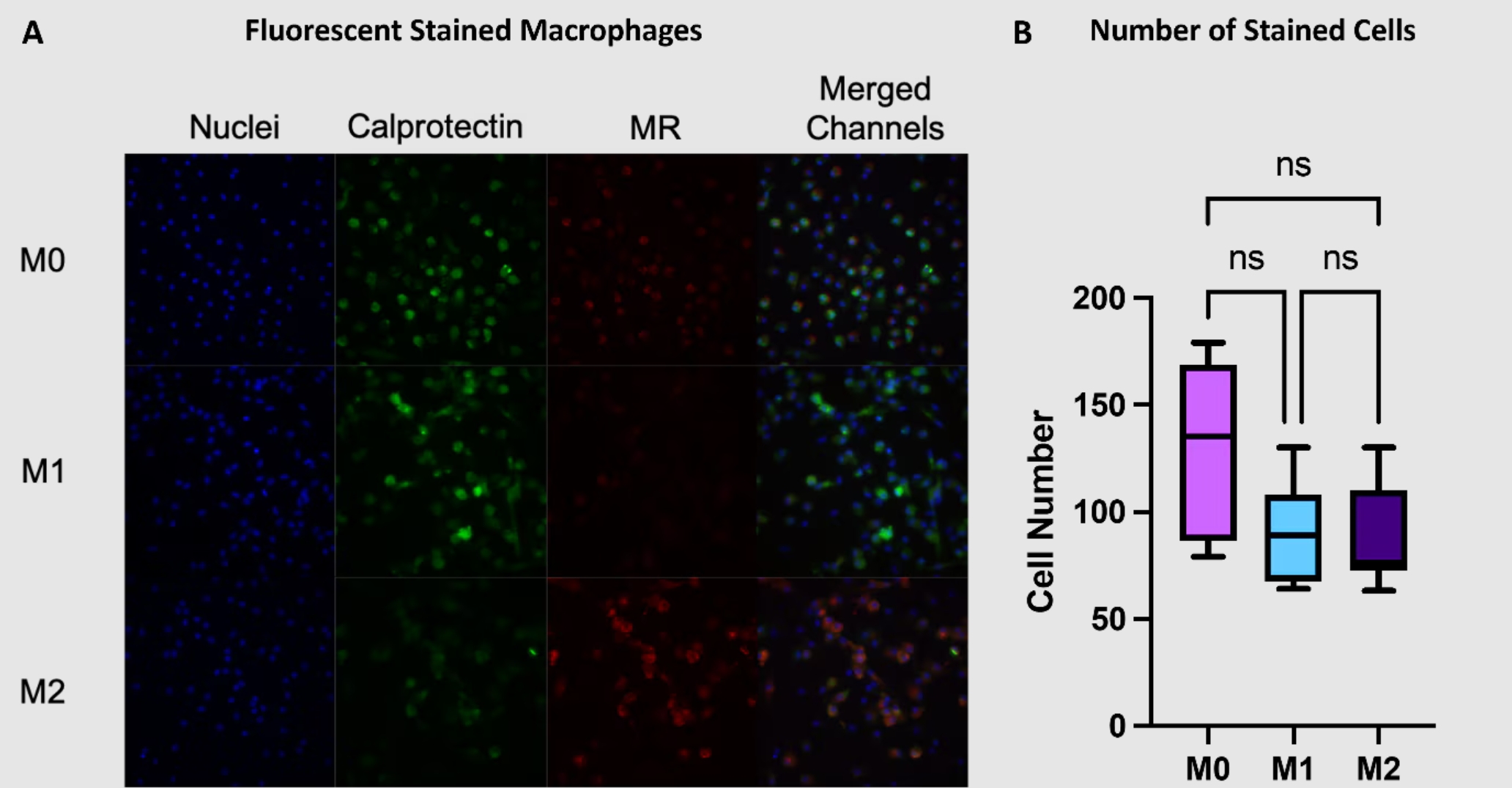
図3. (A)マクロファージの免疫蛍光染色画像。カルプロテクチン(緑)、MR(赤)、核(青:DAPI染色)とそのmerge画像。(B)各画像の細胞数。平均値±SD(n=3)。
c. 炎症性サイトカインと抗炎症性サイトカインの定量
細胞から分泌されたサイトカインを定量する場合は、培地を回収して所定の条件で測定します。各種ELISAキットを使用すると、マクロファージのサイトカインプロファイルを測定して表現型を同定できます。 図4には、サイトカインを定量した実例を示しています。炎症性サイトカインとしてTNF-α、IL-6を、抗炎症性サイトカインとしてIL-10、CCL18を指標とし、培地中の各サイトカイン濃度を測定しました。
プロテインテックの関連製品:ELISAキット検索
TNF-α ELISAキット
IL-6 ELISAキット
IL-10 ELISAキット
CCL18関連製品
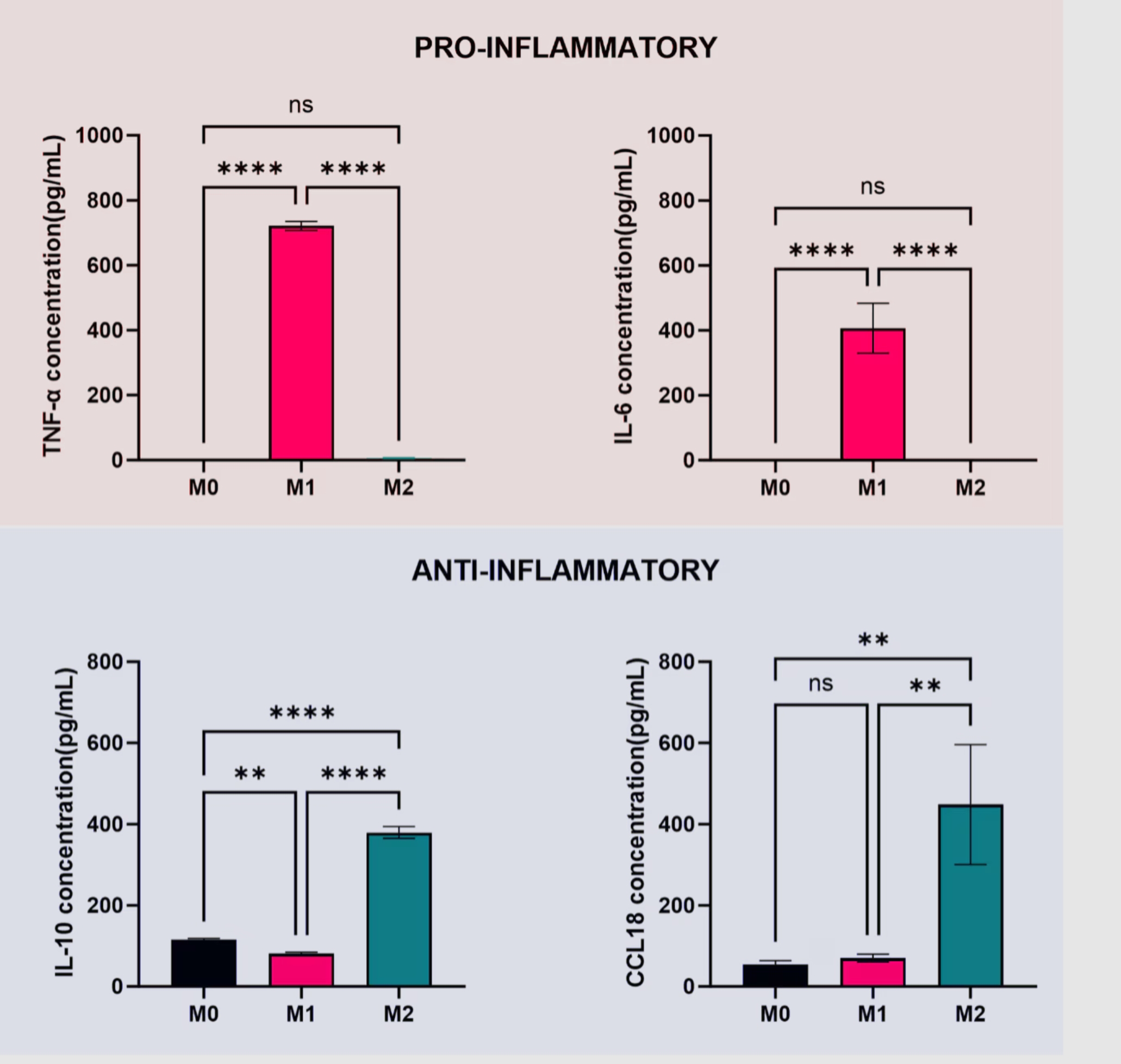
図4. マクロファージによって分泌された培地中のサイトカインTNF-α、IL-6、IL-10、CCL18を定量しました。平均値±SD(n=3)。
終わりに
マクロファージは病原性微生物の排除、細胞デブリの除去、創傷治癒等の働きにより、恒常性の維持に極めて重要な役割を果たしています。反面、マクロファージは腫瘍の増殖や異物反応の進行を刺激する場合もあります。
したがって、マクロファージの分極化や分極機構の解明は、この免疫細胞の有益な作用に働きかけると同時に有害な側面を抑える治療戦略・新規素材を開発するうえで極めて重要となります。
プロテインテックの関連製品
|
ターゲット |
||||
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
|
|
|
|
✔️ |
✔️ |
|
✔️ |
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
✔️ |
|
|
|
✔️ |
✔️ |
|
|
|
|
✔️ |
|
|
|
|
|
✔️ |
|
|
|
著者略歴
|
2020年、Milan大学(イタリア)Pharmaceutical Chemistry and Technology修士号取得(110/110 cum Laude)。最終学年では、医薬品化学の研究プロジェクトに携わり、抗マラリア薬の候補物質である共有結合性GAPDH阻害剤の設計・合成を行いました。 |
Consuelo Coser(Nottingham大学、博士課程在籍) |
参考文献
1 DD Chaplin. Overview of the immune response. J Allergy Clin Immunol. 2010 Feb;125(2 Suppl 2):S3-23.
5 X Huang, et al. Polarizing Macrophages In Vitro. Methods Mol Biol. 2018:1784:119-126.