逆相タンパク質アレイ—ハイスループットなプロテオミクスツール
逆相タンパク質アレイとは?ウェスタンブロット・ELISA・質量分析法との違いとメリット
逆相タンパク質アレイ(RPPA:Reverse Phase Protein Array)は多数のサンプル(~1500検体)を同時定量可能な、抗体を利用するハイスループットなプロテオミクス研究手法です。RPPAの使用例としては、臨床検体中のバイオマーカーの定量、リン酸化によるシグナル伝達相互作用のマッピング、分子標的薬のような薬剤のターゲットになる分子の評価等が挙げられます。RPPAには、ウェスタンブロット(WB:Western blot)、質量分析(MS:Mass spectrometry)、ELISA等のその他のプロテオミクスの手法と比較して優れた利点が数多く存在します。
|
目次 RPPAのワークフロー |
RPPAのワークフロー
RPPAに使用するサンプルは、ウェスタンブロットのサンプル調製と同様にSDS溶液への溶解と加熱変性により調製します。こうして調製したライセート状のサンプルを、384または1536ウェルのマルチウェルプレートに添加します。次に、濃縮サンプルの高い粘度にも対応可能なソリッドピン方式(solid pin system)のマイクロアレイヤーで、メンブレンがコーティングされたガラススライド上にサンプルをプリントし、メンブレン上に「ドット」を作成します。サンプルは3回測定し、多くの場合標準曲線作成用の段階希釈サンプルも測定します。スライドにブロッキング処理を施して、一次抗体をプローブとしてインキュベーションします。一次抗体の検出にはHRP標識二次抗体や蛍光標識二次抗体を使用しますが、タンパク質の存在量が少なく「ドット」は微小であるため、多くの場合シグナルを増幅するステップを実施する必要があります。その後、明視野観察による発色シグナル(DAB染色の場合)、発光シグナル、または蛍光シグナルのいずれかのシグナルを検出します。シグナル検出と定量は機器によって自動的に処理され、データはソフトウェア上でそのまま解析できます。RPPAのワークフローの概要は図1をご覧ください。
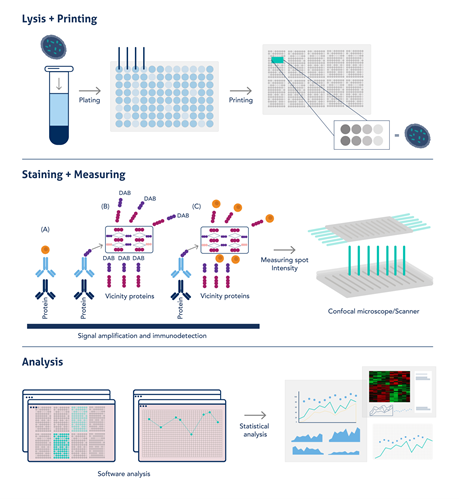
図1. RPPAの簡略なワークフロー(図はAkbani et al, 2014より引用・編集。PMID:24777629)
RPPAの利点
- RPPAは、抗体によるシグナル増幅効果が高く(DABによる発色、化学発光または蛍光シグナルを検出します)検出感度が高いため、微量のタンパク質からデータを取得できます。
- シグナルの読み取りと定量が自動化されているため、スループットが向上し、ヒューマンエラーが低減されます。
- 動物種の異なる一次抗体を組み合わせることで、マルチプレックス検出の選択肢が広がります(例:マウス抗体+ウサギ抗体)。
- 膨大な数の検体を一括して読み取れるので測定シグナルの均一性が確保され、同一タンパク質のシグナルの変化を検体間で高精度に比較できます。
代表的なタンパク質解析ツールとの比較
- ウェスタンブロットは、SDS-PAGEやメンブレンへのトランスファー等の工程が多く、1回の実験で同時に比較できるサンプル数が限られます。解析には30~50 μgのタンパク質ライセートが必要で、サンプルによっては抽出量の確保が課題になります。一方、RPPAは1サンプルあたり約5 μgのタンパク質を抽出できれば解析が可能です。
- 質量分析は、ウェスタンブロットとは原理や手法が異なる中核的なプロテオミクス解析ツールで、サンプル中の広範なタンパク質を一度に定量できると同時にタンパク質アイソフォームも検出可能な点にメリットがあります。既知の少数のターゲットを高感度に定量する場合と網羅的に新規タンパク質を同定する場合では最適なモードや条件が異なり、両立は困難とされています。質量分析によるプロテオミクス解析は、新規タンパク質を同定する際に強みを発揮します。しかし、全長タンパク質ではなくペプチド断片を対象とするケースが多く、翻訳後修飾の変化等は検出・解析できるものの専門知識が求められ、細胞のシグナル伝達の研究への応用には工夫を要します。既知のターゲットを調べたい場合、RPPAは非常に高感度で費用対効果に優れています。また、質量分析法はサンプル前処理に多くの工程を要し、一度に処理できるサンプル数が限られています。
- ELISAは、高感度で中程度のスループットの免疫学的タンパク質解析法ですが、主に溶液中に溶解したタンパク質を検出します。RPPAは組織/生検サンプルだけでなくFFPEサンプル(ホルマリン固定パラフィン包埋サンプル)のタンパク質も検出可能であるため、前述のタンパク質解析ツールよりも臨床用途のハイスループットアッセイに適しています。ELISA(特にサンドイッチELISA法)では、標的タンパク質の異なるエピトープを認識する捕捉抗体と検出抗体の抗体ペアを選定し、その組み合わせや条件を検討する必要があります。RPPAは基本的に1種類の一次抗体を使用して検出する形式で、ウェスタンブロットで特異性が立証された抗体を選定し、シグナル特性等を評価して基準を満たした抗体を用いて実際に測定を行います。
研究手法の一覧表
| 手法 | 長所 | 短所 |
|---|---|---|
|
ウェスタンブロット |
|
|
| ELISA |
|
|
| 質量分析 |
|
|
| 免疫組織化学染色(IHC)・免疫蛍光染色(IF) |
|
|
| 逆相タンパク質アレイ(RPPA) |
|
|
高品質なデータの鍵を握る一次抗体のクオリティ
RPPAでは、分子量ごとに分離されていない状態のタンパク質ライセートに抗体をプロービングして目的タンパク質を検出します。そのため、大規模アッセイに先立ち、抗体が正しいタンパク質を検出できるのか予備実験で検証し、抗体の信頼性を担保しなければなりません。ポジティブコントロールとネガティブコントロールを設定してウェスタンブロットを実施し、対象タンパク質に対する抗体の特異性を確認することが推奨されます。
プロテインテック抗体の使用実績
2022年にJournal of Immunology誌に発表された論文(Fan et al. 2022、PMID:36150727)では、ヒトマクロファージとex vivo肺がん器官型培養(OTC)モデルにおける代謝経路の切り替わりが果たす役割が検証されています。マクロファージはその分極化状態に応じて正反対の働きを示し、この違いは腫瘍微小環境に反映されます。腫瘍関連マクロファージ(TAM:Tumor-associated macrophage)は、典型的なM2マクロファージ(代替活性化マクロファージ)様の表現型を示し、T細胞のダウンレギュレーションを介する免疫抑制的な環境の構築によって腫瘍増殖を促進するマクロファージです。この状態は特に非小細胞肺癌(NSCLC:Non-Small Cell Lung Carcinoma)に顕著で、T細胞が排除された領域が存在することで、免疫チェックポイント阻害薬への抵抗性を獲得すると考えられています。
著者らは、安定同位体トレーサーと逆相タンパク質アレイ(RPPA)を使用して、様々な分極化誘導処理や酵母由来β-グルカン粒子(WGP)に反応して生じる、各マクロファージや腫瘍関連マクロファージの経時的な免疫調節性代謝変化を追跡しました。
RPPAはハイスループットなタンパク質定量を実施可能な、抗体を利用するマイクロアレイ技術です。この論文で著者らがRPPAに使用した一次抗体のうち、約80%がプロテインテックの製品でした(この1つの論文に42種類のプロテインテックの抗体が使用されました)。プロテインテックは、マクロファージの代謝を経時的にマッピングする大規模な取り組みを支援できたことを非常に意義深いものと受け止めています。今後も研究者の皆様の成果を紹介できるよう、皆様の研究を支えてまいります。
論文に使用されたプロテインテックの抗体
|
1 |
19677-1-AP |
|
|
2 |
60006-1-Ig |
|
|
3 |
16131-1-AP |
|
|
4 |
25949-1-AP |
|
|
5 |
17762-1-AP |
|
|
6 |
12842-1-AP |
|
|
7 |
11375-1-AP |
|
|
8 |
14299-1-AP |
|
|
9 |
22104-1-AP |
|
|
10 |
10566-1-AP |
|
|
11 |
20960-1-AP |
|
|
12 |
13333-1-AP |
|
|
13 |
66197-1-Ig |
|
|
14 |
15932-1-AP |
|
|
15 |
13268-1-AP |
|
|
16 |
15365-1-AP |
|
|
17 |
16806-1-AP |
|
|
18 |
66142-1-Ig |
|
|
19 |
66146-1-Ig |
|
|
20 |
60269-1-Ig |
|
|
21 |
66196-1-Ig |
|
|
22 |
12855-1-AP |
|
|
23 |
55013-1-AP |
|
|
24 |
15904-1-AP |
|
|
25 |
15212-1-AP |
|
|
26 |
16588-1-AP |
|
|
27 |
16754-1-AP |
|
|
28 |
14892-1-AP |
|
|
29 |
21829-1-AP |
|
|
30 |
13763-1-AP |
|
|
31 |
14718-1-AP |
|
|
32 |
60268-1-Ig |
|
|
33 |
55380-1-AP |
|
|
34 |
15851-1-AP |
|
|
35 |
25174-1-AP |
|
|
36 |
10620-1-AP |
|
|
37 |
21898-1-AP |
|
|
38 |
60291-1-Ig |
|
|
39 |
19003-1-AP |
|
|
40 |
25413-1-AP |
|
|
41 |
19987-1-AP |
|
|
42 |
18068-1-AP |
Lucie Reboud著(マンチェスター大学、博士課程4年、プロテインテックScience Marketingインターン生)



